Hydrated ionic liquids enhance long-term stability of structure and functionality in membrane proteins
Innovative technology that paves the way for new drug discoveries and biodevice development
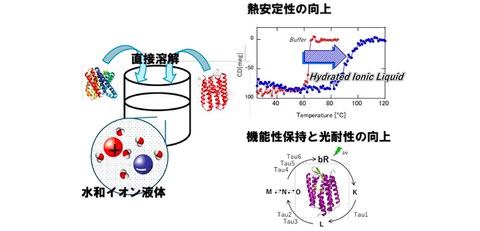
- The research group succeeded in preserving the structure and functionality of membrane proteins by direct dissolution.
- Thermal stability improved by more than 20°C, and light-driven proton transfer protein demonstrated ten times greater light resistance while retaining its function.
- This technology is expected to be a game-changer in drug discoveries targeting membrane proteins and in the field of biodevices.
Outlines
A collaborative research team including Associate Professor (Lecturer) Eiichi Mizohata of the Graduate School of Engineering at the University of Osaka, Senior Assistant Professor Kyoko Fujita of the School of Pharmacy at Tokyo University of Pharmacy and Life Sciences, and Associate Professor Yuji Furutani of the Opto-Biotechnology Research Center at Nagoya Institute of Technology has succeeded in developing a new technology that directly dissolves membrane proteins in hydrated ionic liquids, achieving both structural and functional maintenance that have been considered difficult to achieve until now. Membrane proteins are important molecules that are the targets of approximately 60% of pharmaceuticals, but their instability has been a major obstacle to research and development. This technology is expected to significantly advance drug discovery and the development of next-generation biosensors.
Research Background
Membrane proteins are in cell membranes and play a key role in vital activities such as transporting substances and transmitting information, making them one of the most vital targets in the fields of medicine, drug discovery, and biotechnology However, because they are delicate molecules whose structure and function are easily impaired outside the body, they are difficult to handle stably, which has been a bottleneck in research and application. Conventional methods using buffers and surfactants have limitations, and the development of new technology that can achieve fundamental stabilization was demanded.
Research Contents
In this study, by using hydrated ionic liquids, which are liquid salts containing a small amount of water, the researchers were able to directly dissolve transmembrane proteins (TehA and bacteriorhodopsin) and successfully stabilize them while maintaining their structure and functionality.
Due to their instability, membrane proteins are difficult to handle in conventional buffer solutions. However, this study revealed that the constituent ions (cations and anions) and water content of hydrated ionic liquids have a significant effect on structural retention and stability. In particular, it was elucidated that a hydrated ionic liquid using cholinium dihydrogen phosphate can dissolve membrane proteins while maintaining their higher-order structure, and can increase the thermal denaturation temperature by more than 20°C.
In general, it is known that increasing the thermal denaturation temperature improves thermal and long-term stability. The improvement in structural stability achieved by this technology is extremely significant.
Furthermore, because differences in water content significantly affect the structure and stability of proteins, it is suggested that the number and properties of water molecules present in biological membranes play a key role in maintaining the function of membrane proteins.
In addition, by irradiating bacteriorhodopsin (bR), which has light-driven proton transfer ability, with laser light and observing the formation of intermediate states related to proton transport, maintenance of its functionality was also demonstrated. In addition, improved functional stability has been confirmed, such as approximately 10 times greater resistance to photobleaching due to laser irradiation.
These results have great potential for drug discovery and biodevice development as a new method for more easily and accurately analyzing the structure and functional evaluation of membrane proteins.
Overview of the research
Credit: Eiichi Mizohata
Future Developments
The method of stable dissolution for membrane proteins established in this study is expected to greatly accelerate the research on membrane protein, which has previously been hindered by their difficulty of handling. Especially, it is expected to be widely applied as a promising option for drug discovery screening targeting membrane proteins, biosensor development utilizing its functionality, and efficient structural analysis of membrane proteins.
This technology also has higher practicality from the perspective of Target Product Profile (TPP) in drug discovery. For example, the long-term storage stability and thermal stability (improved above 20°C) of membrane proteins are directly linked to quality maintenance during formulation and transportation, and also contribute to improving stability over time. Furthermore, maintaining stable membrane protein functionality contributes to improving the reproducibility of functional evaluation and screening.
Because hydrated ionic liquids have low volatility and are easy to prepare, they can also be used to stabilize formulations. This will be a new option for achieving both structural stability and functionality in the design, evaluation, and manufacturing processes of pharmaceuticals that target membrane proteins.
This technology can be applied not only to basic research but also to applications from the early stages of the drug discovery process (target verification and screening) to later stages of development (formulation and quality control). As a game-changer in the development of drugs targeting membrane proteins, it is expected to contribute to the promotion of innovative research in the fields of life science, medicine, and engineering.
Notes
The article, “Hydrated ionic liquids enhance stability and preserve functionality in transmembrane proteins,” was published in International Journal of Biological Macromolecules at DOI: 10.1016/j.ijbiomac.2025.148096
