\Does stress make the skin younger!?/ Elucidated the mechanism of skin youthfulness using African turquoise killifish
Endoplasmic reticulum (ER) stress restores skin stem cells to a youthful state
- Although endoplasmic reticulum (ER) stress has been thought to be a defense mechanism that works when cells are subjected to stress, the research group discovered that aged epidermal stem cells can be restored to a younger state.
- Research into the aging mechanism requires a long time, so its detailed understanding has not yet progressed much, however, by combining African turquoise killifish with unique visualization and comprehensive analysis techniques, elucidating the mechanism in a short period of time has been achieved.
- It is expected to develop promising therapeutic and preventive strategies for human skin aging.
Outlines
A research group including a graduate student Daniel Semmy (doctor course, Graduate School of Frontier Biosciences), Assistant Professor Kota Abe, and Professor Tohru Ishitani at Research Institute for Microbial Diseases, the University of Osaka, in collaboration with Kyushu University, Kumamoto University, and others, has clarified for the first time in the world that the endoplasmic reticulum (ER) stress contributes to maintaining the youthfulness of skin stem and progenitor cells.
Aging research has been slow to progress because of the long lifespan of conventional vertebrate models (approximately 3 years for mice and zebrafish), meaning that analysis takes years, and there is a lack of technology that can visualize and comprehensively analyze changes in cells at molecular level. In this study, the researchers used the African turquoise killifish (abbreviated as killifish), a super-fast-aging fish that is a vertebrate like humans but ages and dies within two to three months of sexual maturity, as a model to analyze the mechanism of skin aging by combining visualization technology developed by the team with comprehensive analysis technology for gene expression dynamics. As a result, they discovered that the endoplasmic reticulum (ER) stress-response pathway, which is well known as a defense mechanism that works when cells are under stress, is surprisingly highly activated in the epidermal basal layer, controlling gene expression and maintaining the "youth" (proliferative activity) of epidermal stem cells. Furthermore, if ER stress is applied to aged epidermis, gene expression in epidermal stem cells is reverted to a younger state and restores their proliferation activity. Also, analysis of mouse tissues and publicly available human data strongly suggested that, as in killifish, stem cell control and age-related functional decline through the ER stress-response, Vcp pathway may occur in human and mouse skin as well.
These results elucidated that the ER stress-response pathway, which has been thought to be a defense mechanism against stress, maintains the youthfulness of the skin, and provides promising insights that could develop new therapeutic and preventive strategies that could rejuvenate aged epidermal stem cells.
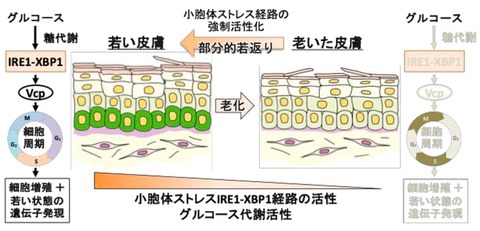
Fig. 1 Endoplasmic reticulum (ER) stress maintains youthful skin
Credit: Tohru Ishitani
Research Background
Aging of skin epidermal stem cells: Tissue and organ homeostasis is supported by stem cell activities. For example, in the epithelium that protects the living body from injury and infection, self-renewing epidermal stem cells are located in the basal layer (the lowest layer epidermis) and play an important role in the continuous cell metabolism of skin tissue and wound repair. However, epidermal stem cells lose their ability to proliferate with age, resulting in a decline in skin homeostasis and their ability to repair damage. Although the mechanisms of epidermal stem cell proliferation/differentiation, and skin aging have been widely studied, it is still unclear what level of molecular changes within stem cells drive the functional decline in stem cell with age.
The ER stress-response and aging: In addition to stem cell aging, another major factor in aging is known to be the breakdown of protein homeostasis (proteostasis). Proteostasis is a system that monitors protein synthesis, ensures protein quality control, and uses adaptive mechanisms to mitigate the accumulation of unfolded and misfolded proteins, thereby preventing the accumulation of abnormal proteins and maintaining homeostasis of tissues. The ubiquitin-proteasome and lysosome-autophagy systems are major players in proteostasis maintenance, eliminating unfolded proteins from cells through proteolysis. These systems are known to become less active with aging. In addition to these two, there is a third system, the endoplasmic reticulum (ER) stress-response. The endoplasmic reticulum (ER) is responsible for the synthesis and folding of approximately one-third of the total proteins in the cell. Stresses such as oxidative stress, the appearance of mutant proteins, and hypoxic conditions induce excessive accumulation of misfolded proteins (denatured proteins) in the endoplasmic reticulum. If this ER stress continues, it can cause endoplasmic reticulum dysfunction and cell death. In order to avoid this, the ER stress-response is activated in cells where the stress occurs and is handled by three pathways: the Ire1-Xbp1s pathway, the (Perk)–activating transcription factor 4 (Atf4) pathway, and the Atf6 pathway, which prevent the accumulation of denatured proteins. Regarding the relationship between ER stress and aging, it has been reported that the response ability toward the ER stress-response pathway in the invertebrate nematode decreases with age. However, the age-related changes in the ER stress-response in vertebrates are not yet fully understood. One of the reasons for this is the long lifespan of conventional vertebrate models (approximately 3 years for mice and zebrafish), making analysis time too long.
A new animal model to accelerate aging research: In this study, the researchers used the African turquoise killifish (abbreviated as killifish), a super-fast-aging fish that is a vertebrate like humans but ages and dies within two to three months of sexual maturity, as a model to research on aging. Last year, they used killifish to identify "The mechanism that makes females live longer than males" and "Vitamin D can extend healthy lifespan" (Abe et al., Science Advances 2024). However, because killifish are a new model animal, the development of experimental techniques has been slow. For example, in recent years, "spatial transcriptomics" has been a method for comprehensively analyzing changes in the state of cells locally within tissues at the molecular level. However, since most of this technology is currently developed commercially, it can only be used on representative human and mouse tissues (samples with many users and that are commercially viable). To conduct research in killifish at the same depth as in mice, spatial transcriptomics must be achieved in killifish.
Research Contents
Visualizing the ER stress-response with killifish: In order to investigate age-related changes in the ER stress-response in a short period of time, the research group decided to use killifish, which ages rapidly, as a model (Fig. 2, top). Specifically, they first focused on the Ire1-Xbp1s pathway, which is highly conserved from invertebrates to humans, and developed a killifish visualizing the activity of this pathway. By using analysis of this visualized killifish and other methods, they found that the Ire1-Xbp1s pathway is activated with age in various tissues, including the liver, heart, and brain. Indeed, accumulation of misfolded proteins and changes in the shape and size of the endoplasmic reticulum, signs of ER stress, were also observed in aged livers. In other words, it has become clear that chronic ER stress occurs in the liver and other organs as a result of the accumulation of misfolded proteins due to aging. This result that "chronic stress occurs with aging" resulted in as expected.
The endoplasmic reticulum (ER) stress-response promotes proliferation of epidermal stem cells:
However, as the research group continued the visualization analysis, something unexpected was discovered. This means that the Ire1-Xbp1s pathway is activated in the epidermal basal layer, where epidermal stem cells reside at a younger age, and its activity decreases with age (Fig. 2, bottom). Interestingly, no misfolded protein was detected in cells of the basal layer. This suggests that the strong activation of the Ire1-Xbp1s pathway in the basal layer may not be related to the accumulation of misfolded. Then, what does the Ire1-Xbp1s pathway do in the young basal layer? As mentioned above, young stem cells have higher rate of proliferative activity. In fact, in young epidermis, basal layer cells (stem cell group) with high Ire1-Xbp1s activity had high proliferation activity (Fig. 3, top). Furthermore, in aged epidermis, where Ire1-Xbp1s activity was reduced, proliferation activity was lost (Fig. 3, middle panel). Interestingly, when tunicamycin, a drug that activates ER stress, was administered to aged fish, cell proliferation activity in the epidermal basal layer was restored (Fig. 3, bottom). These results suggest that the activity of the ER stress-response pathway in epidermal basal layer promotes cell proliferation and that forced activation of ER stress may rejuvenate the activity of the aged epidermal basal layer. They also confirmed that disrupting the Ire1 gene in killifish reduces the proliferation of epidermal basal layer cells at a young age, and that treating cultured epidermal stem cells extracted from young killifish with tunicamycin stimulates cell proliferation, while administering a drug that inhibits Ire1 reduces proliferation. In other words, it has become clear that the Ire1-Xbp1s pathway contributes to the youthfulness (proliferative activity) of epidermal stem cells.
Spatial transcriptomics reveals the regulation of youth by ER stress: How does the ER stress pathway change the state of epidermal basal layer cells? In order to investigate this, it is necessary to analyze age-related changes in gene expression in epidermal basal layer cells. However, there was no such analytical method available for the local cells of the epidermal basal layer, let alone for the special organism known as the killifish. However, Professor Ishitani was fortunate to receive support from his research colleagues of the same generation, Dr. Yasuyuki Ohkawa (currently Director, Medical Institute of Bioregulation, Kyushu University) and Dr. Shinya Oki (currently Professor at the Graduate School of Medicine at Kumamoto University), who used to work on the same campus at Kyushu University. This gave the professor the opportunity to use the cutting-edge spatial transcriptomics technology they developed, photo-isolation chemistry (PIC), on killifish. PIC allows researchers to obtain transcriptome information by illuminating cells of interest with light without disassembling tissue. Compared to conventional techniques, it has the advantage of being inexpensive and applicable to any living species. They performed PIC analysis of killifish epidermal basal layer cells and confirmed that the gene expression patterns (transcriptome) were clearly different between young and aged epidermal basal layer cells, and that administering tunicamycin (ER stress) to aged epidermal basal layer cells rejuvenated these patterns (Fig. 4). Importantly, tunicamycin treatment not only reversed the age-related decline in gene expression related to ER stress-response and cell proliferation but also canceled the age-related changes in gene expression related to Notch signaling and Plexin-Semaphorin signaling, which contribute to the regulation of epidermal stem cell differentiation. In other words, it has become clear that ER stress rejuvenates various activities in the aged epidermal basal layer.
Ire1-Xbp1s promotes proliferation through Vcp: How does the Ire1-Xbp1s pathway promote cell proliferation? The researchers focused on genes whose PIC analysis showed high expression in young ages, decreased expression with aging, and restored expression by tunicamycin treatment. As a result of these analyses, they found that Vcp, which positively regulates cell cycle progression, is induced to express downstream of the Ire1-Xbp1s pathway, promoting the proliferation of epidermal basal layer cells (Fig. 1).
Decreased glucose metabolism leads to age-related decline in the Ire1-Xbp1s activity: Next, they investigated the mechanism by which the Ire1-Xbp1s activity declines with age. First, by PIC analysis and metabolite measurement, they found that glucose metabolic capacity in epidermal basal layer declines with age. Furthermore, it has been found that adding glucose to cultured killifish epidermal stem cells activates the Ire1-Xbp1s pathway and promotes cell proliferation. In other words, it was suggested that the decline in glucose metabolism associated with aging may lead to a decrease in the Ire1-Xbp1s activity and cell proliferation in epidermal basal layer (Fig. 1).
Similar regulation also occurs in humans and mice: Thus, the research group found that in killifish, the glucose metabolism- Ire1-Xbp1s-Vcp pathway maintains stem cell activity in the young epidermal basal layer, and that an age-related decline in glucose metabolism leads to a decrease in the activity of this pathway and a decline in the function of epidermal stem cells. So what about mammals? First, through mouse skin tissue analysis in collaboration with the Institute of Medical Science, the University of Tokyo, it has been confirmed that the Ire1-Xbp1s pathway is activated in the basal layer of mice at a young age, and that its activity decreases with age. Furthermore, analysis of publicly available transcriptome data for human skin revealed that, similar to killifish skin, the expression of ER stress response-related genes, Vcp genes, and glucose metabolism-related genes decreases in an age-dependent manner. Furthermore, they confirmed that tunicamycin can promote proliferation in human skin cells (keratinocytes), and that inhibition of the Ire1-Xbp1s pathway can suppress proliferation. In other words, it was suggested that the same stem cell control and age-related functional decline that occur in killifish skin also occur in human and mouse skin.
Fig. 2 The Ire1-Xbp1s pathway decreases its activity with age in epidermal stem cells
Credit: Tohru Ishitani
Fig. 3 The Ire1-Xbp1s pathway supports the proliferation of epidermal stem cells
Credit: Tohru Ishitani
Fig. 4 ER stress rejuvenates gene expression patterns
Credit: Tohru Ishitani
Social Impact of the Research
The results of this study have clarified a new, previously unknown aspect of the ER stress-response, which is known as a defense system activated when cells are under stress, maintaining the youthfulness of skin.
This control may function not only in skin but also in stem cells of other tissues, and it is expected to lead to the elucidation of an unknown universal mechanism for controlling stem cell aging.
This study also demonstrated a new approach, "inflicting ER stress," that may potentially rejuvenate aged skin stem cells. A deeper understanding of the role and regulation of the ER stress response in human epidermal stem cells is hoped to lead to the development of new, promising treatment and prevention strategies for human skin aging based on this discovery.
In addition, the new epidermal cell proliferation-promoting factor Vcp discovered in this research shows that its expression in human skin decreases in men from their 20s to 40s, but remains unchanged after the age of 40. In contrast, its expression gradually decreases in women from their 40s onwards. Interestingly, while skin aging occurs earlier in men, it progresses rapidly in women after menopause, and it may be related to gender differences in aging. Now, the researchers are not only using super-fast-aging fish and human data to discover new mechanisms of aging and youth maintenance but also analyzing gender and individual differences in these mechanisms, continuing their research with the aim of "individual aging control" in the future. Please look forward to the next outcomes.
Notes
The article, “ER stress Ire1-Xbp1s pathway maintains youthful epidermal basal layer through the regulation of cell proliferation,” was published in British scientific journal of Aging Cell at DOI: https://doi.org/10.1111/acel.70258.

