Confirmed safety and effectiveness of new cancer treatment using astatine, a targeted α-therapy drug
Investigator-initiated clinical trials for refractory thyroid cancer
- An investigator-initiated clinical trial (first-in-human study) was conducted on patients with refractory thyroid cancer using astatine, a new cancer treatment drug that releasesα-radiation from inside the body, and confirmed the safety of the treatment and the high therapeutic effect in the high-dose group (a reduction of tumor markers of more than 50% and disappearance of lesions on diagnostic imaging).
- Even when the current standard treatment using radioactive iodine (131I) is ineffective, α-radiation, which emit high energy over a short range, can target cancer cells and provide targeted treatment, and this eliminates the need for hospitalization in a special room, making it a more patient-friendly cancer treatment.
- Since astatine can be produced using Japanese accelerator, the aim is to build a domestic network for supplying a targeted α-therapy drugs for various cancers in the future.
Outlines
A research team including Associate Professor (Lecturer) Tadashi Watabe, Professor Noriyuki Tomiyama (Diagnostic and Interventional Radiology), Assistant Professor Kosuke Mukai, Endowed Chair Associate Professor Atsunori Fukuhara, Professor Iichiro Shimomura (Department of Metablic Medicinem) from the Graduate School of Medicine of the University of Osaka, and Laboratory Head Hiromitsu Haba from the RIKEN Nishina Center for Accelerator-Based Science, conducted an investigator-initiated first-in-human clinical study using astatine, a new targetedα-therapy drug, in patients with refractory thyroid cancer at the University of Osaka Hospital.
Over a period of approximately three years from 2022 to 2024, 11 patients with thyroid cancer who had not responded to standard treatments were administered a single administration dose of sodium astatide ([²¹¹At]NaAt) injection, and the safety and efficacy were evaluated. The results showed that this therapy drug can be administered safely, and furthermore, in the medium and high dose group (nine patients), three patients showed a 50% or greater reduction in tumor markers, and in three patients, radioactive iodine (¹³¹ I) imaging confirmed the disappearance of metastatic lesions in the liver and bones (one patient showed complete disappearance, and two patients showed near-complete disappearance).
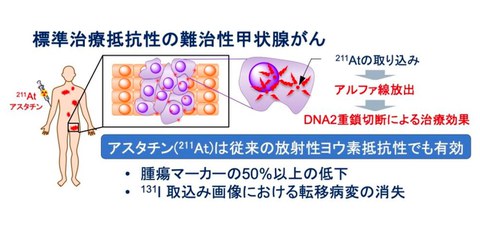
The standard treatment for differentiated thyroid cancer is radioactive iodine (¹³¹ I), but even with multiple treatments, sufficient therapeutic effects may not be achieved. On the other hand, astatine is an element with properties very similar to iodine, and emits short-range, high-energy radiation calledα-radiation, which allows it to attack cancer cells precisely without affecting surrounding normal cells. This feature is expected to resolve the conventional issues faced by refractory thyroid cancer while also providing more effective treatment (Fig. 1).
Going forward, Alpha Fusion Inc. (a startup from the University of Osaka), the intermediary company, will conduct corporate clinical trials and continue to evaluate safety and efficacy, with the aim of obtaining approval as a pharmaceutical. In the future, unlike existing 131I treatments, this is expected to become a patient-friendly cancer treatment that can be administered on an outpatient basis, without requiring hospitalization in a dedicated room.
Fig. 1 Treatment using astatine (211At), anα-therapy drug
(By accumulating it in metastatic lesions throughout the body, α-radiations are emitted from inside the body)
Credit: Tadashi Watabe
Background of the Clinical Trial
Most thyroid cancers are called differentiated thyroid cancers and can absorb iodine. Currently, treatment for metastatic or recurrent differentiated thyroid cancer involves hospitalization in a special room and treatment with radioactive iodine (131I). However, repeated treatments may not always be effective enough, and there is a lack of dedicated hospital rooms. Furthermore, the molecular-targeted agents used in treatment-resistant patients require continuous daily oral administration, and its side effects may make it difficult to continue treatment. Unlike β-particles emitted by conventional radioactive iodine (131I), α-radiation emitted by astatine have a short range and emit high energy, making it possible to target and treat cancer cells without affecting surrounding normal tissue. Astatine can be produced using an accelerator (cyclotron), and is attracting worldwide attention, with the University of Osaka becoming one of the world's leading astatine research centers.
The University of Osaka has developed sodium astatide ([²¹¹At]NaAt) injection as a new treatment for refractory thyroid cancer, and the RIKEN’s Radioactive Isotope Beam Factory (RIBF) has developed the technology to mass-produce the raw astatine material and is providing a stable supply to the university. The University of Osaka Hospital has also established a Good Manufacturing Practice (GMP) manufacturing system for investigational drugs using automatic separation and purification system.
Contents of the Clinical Trial
A first-in-human investigator-initiated phase 1 trial (Alpha-T1 trial) was conducted as an administration dose study to confirm the safety, pharmacokinetics, and efficacy of sodium astatide ([211At]NaAt), α-therapy drug, in patients with refractory differentiated thyroid cancer who have not shown sufficient therapeutic effects with standard treatments such as radioactive iodine (131I). Many of the patients had undergone three or more prior 131I treatments without achieving satisfactory therapeutic effects and had multiple metastatic lesions in the lungs and bones. Starting with a low dose (1.25MBq/kg), the dose was gradually increased to 2.5MBq/kg and 3.5MBq/kg, and a total of 11 patients received a single administration dose.
Strict follow-up observation was conducted for six months after administration, and although temporary nausea was observed immediately after administration, no serious side effects were observed, confirming that the drug can be administered safely. Regarding the therapeutic effect, in the medium- and high-dose groups (2.5 or 3.5 MBq/kg, total of nine patients), tumor marker (thyroglobulin) levels decreased by more than 50% from before the start of treatment in three patients, and imaging diagnostics using radioactive iodine (¹³¹ I) confirmed the disappearance of 131I accumulation in metastatic lesions in three patients (complete disappearance in one patient, and almost complete disappearance in two patients) (Fig. 2). The researchers demonstrated the effectiveness of a targeted α-therapy drug using astatine, even in patients who were resistant to conventional treatments.
In particular, the production of conventional 131I requires a medical nuclear reactor, and Japan is 100% dependent on imports from overseas. In this clinical trial, astatine (211At) was produced using a Japanese accelerator, and it was demonstrated for the first time in the world that its systemic administration as an investigational drug can be effective in treating solid cancers.
Fig. 2 131I-SPECT image after administration of astatine (211At): The lesion has almost completely disappeared (red arrows)
Credit: Tadashi Watabe
Social Impact of the Research
Going forward, Alpha Fusion Inc. (a startup from the University of Osaka), the intermediary company, will conduct corporate clinical trials and continue to evaluate safety and efficacy, with the aim of obtaining approval as a pharmaceutical. In the future, unlike existing 131I treatments, it is expected that this will become a patient-friendly cancer treatment that can be administered on an outpatient basis, without requiring hospitalization in a dedicated room.
In addition, a new cyclotron manufactured by Sumitomo Heavy Industries, Ltd. has been installed in the newly completed TAT Cyclotron Facility at the University of Osaka Research Center for Nuclear Physics, and mass production of astatine will soon begin. In the future, the research group aims to build a supply network that covers the entire Japan, making it possible to administer targeted α-therapy drugs using astatine at medical institutions throughout Japan.
Notes
The article, “First-in-human Study of [²¹¹At] NaAt as Targeted Alpha Therapy in Patients with Radioiodine-Refractory Thyroid Cancer (Alpha-T1 Trial),” was published in Journal of Nuclear Medicine at DOI: 10.2967/jnumed.125.270810
