Observing the evolution of magnetic phases through oxygen adsorption
Providing a rationale for molecular device applications via oxygen electron spin
- A new material that can continuously (analogically) variate ferromagnetism (ON) and antiferromagnetism (OFF) depending on the amount of oxygen adsorption on the magnet by directly interacting with the oxygen electron spin of the magnet's properties has been developed.
- For the first time in the world, the research group has achieved continuous observation of the smooth transition of this material from a magnetic ON state to an OFF state close to that of a non-magnetic body as the amount of adsorbed oxygen.
- The result of this research is expected to contribute to the development of next-generation oxygen sensors and high-performance molecular devices as an analog magnet that can respond accurately to the amount of adsorbed oxygen.
Outlines
Oxygen is one of the smallest molecular units that possesses the property of electron spin, which is the origin of magnets. If the oxygen spin can be freely used as an ON/OFF switch for a magnet, it could develop new molecular devices that can selectively detect and control oxygen.
A research group including Associate Professor Wataru Kosaka and Professor Hitoshi Miyasaka of the Tohoku University’s Institute for Materials Research has reported a magnet whose magnetic phase can be switched by adsorbing and desorbing oxygen. However, it was not elucidated how the magnetic phase changes as oxygen adsorption proceeds.
In this study, a joint research team including the forementioned research group, Professor Yasutaka Kitagawa of the Graduate School of Engineering Science at the University of Osaka, and Professor Jun Zhang of Wuhan University, has developed the second porous magnet in which the magnet's ON/OFF states can be controlled by the adsorption and desorption of oxygen gas. The unique characteristic of this new material is that the state of the magnet can be continuously controlled by adsorbing a small amount of oxygen. For the first time in the world, the research team has succeeded in tracking the change of magnetic phase over time, in which a magnet smoothly transitions from an ON state to an OFF state in response to changes in the amount of adsorbed oxygen. This result not only create simple binary ON/OFF switches but also paves the way for the realization of analogically controlled molecular devices.
Research Background
The main components of air are nitrogen, oxygen, argon, and carbon dioxide, of which nitrogen accounts for about 78% and oxygen for about 21%, making up the majority. Oxygen is an essential molecule for living organisms to live. Although its molecular size, boiling point, and other properties are like nitrogen, there are crucial differences between the two molecules, that is magnetism. Oxygen molecules have electron spin and exhibit paramagnetic properties, which are being attracted to magnets and are completely different characteristics from other major gases such as non-magnetic nitrogen and carbon dioxide.
The research group at Tohoku University has focused on this electron spin of oxygen and has been developing new magnetic materials that can directly utilize the magnetic properties of oxygen. The key to realizing functions that are difficult to achieve with conventional magnetic bodies is the flexible structure of the molecules themselves. The research team focused on molecular porous bodies called metal-organic frameworks (MOFs) that consist of metal ions and organic ligands. Not only MOFs are highly structural, but also they are extensible, allowing for the free design of constituent metals and organic molecules, making it possible to utilize the properties of both the lattice and the space, so it will be ideal materials for developing the gas-adsorbing magnets. This research team has developed a number of magnetic materials whose properties change when various gases are adsorbed and desorbed, using porous molecular magnets (MOF magnets) that incorporate the space of MOFs into magnets. This includes materials that directly utilize the electron spin of oxygen. However, the details of how the adsorbed oxygen interacts with the magnet and how the magnetic phase evolves as the amount of adsorbed oxygen increases were not elucidated, and further clarification was demanded.
Research Contents
In investigating its aspect through this collaborative research, the researchers finally discovered a continuous magnetic phase transitions due to a cooperative phenomenon, along with the growth of magnetic domains that correlate with the amount of oxygen adsorption.
The key findings of this study are summarized into three points. First, selective conversion from ferromagnetism to antiferromagnetism has been achieved. The MOF magnets fabricated in this research are ferromagnetic (a Curie temperature (Tc) of 30 K) before gas adsorption. When carbon dioxide is adsorbed, it remains ferromagnetic (the original FM state with Tc = 24 K), but when oxygen is adsorbed, it changes to an antiferromagnetic state, and the magnet becomes OFF state (a Néel temperature (TN) of 28 K). Second, the team observed a continuous magnetic phase transition depending on the amount of adsorbed oxygen. As oxygen adsorption increases, antiferromagnetic domains grow slowly, resulting in a continuous transition from ferromagnetic to antiferromagnetic states. As a result, they observed a smooth shift in the Curie temperature TN from 17 K to 28 K. Third, they have elucidated a new mechanism for magnetic interactions via oxygen spin. Theoretical calculations (DFT calculations) which deal with electronic states based on the crystal structure clarified that the two oxygen molecules adsorbed between the layers establish a new magnetic interaction path via electron spin, causing antiferromagnetism.
Please see the details of the results below.
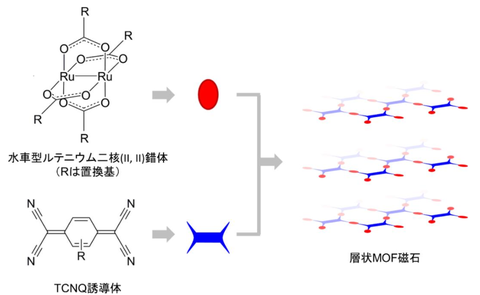
This collaborative research group has previously developed a layered molecular magnet consisting of a carboxylate-bridged paddlewheel-type diruthenium center (II, II) metal complex, which acts as an electron donor molecule, and a TCNQ (7,7,8,8-tetracyano-p-quinodimethane) derivative, which acts as an electron acceptor molecule. (Fig. 1) When the gas adsorption capacity of the compound obtained in this research was examined, it was discovered that it did not adsorb nitrogen, but only carbon dioxide and oxygen. Magnetic measurements demonstrated that the material was ferromagnetic with a Tc = 30 K in the dry state without gas adsorption and remained ferromagnetic (Tc = 24 K) even after carbon dioxide adsorption (Fig. 2). On the other hand, when oxygen was adsorbed, it transformed into an antiferromagnetic state (TN = 28 K) (Fig. 3). Furthermore, by controlling the adsorption rate and gradually changing the amount of adsorbed oxygen, the research group observed a magnetic phase change in which ferromagnetism transitioned to antiferromagnetism, and the TN continuously transitioned from 17 K to 28 K as the amount of adsorption increased (Fig. 3). This is because the antiferromagnetic domains that form upon oxygen adsorption cooperatively control the magnetic order of the entire compound, and this achievement is the first time in the world that a continuous magnetic cooperative phenomenon caused by gas adsorption has been captured.
Structural analysis using single-crystal X-ray crystallography revealed that the framework itself did not change dramatically before and after gas adsorption, and that the adsorbed gas molecules were trapped in pairs in isolated spaces sandwiched between the TCNQ layers (Fig. 4). Although the adsorption environments for carbon dioxide and oxygen are almost the same, magnetic phase evolution occurred only in the case of oxygen, so it can be strongly suggested that the electron spin of oxygen plays a decisive role. DFT calculations clarified that the two oxygen molecules adsorbed between the layers form a new magnetic interaction path between the layers via the oxygen spins, resulting in the progression of antiferromagnetism. This compound is the second in the world to indicate a magnetic phase evolution upon oxygen adsorption. It has ideal characteristics for elucidating the mechanism, such as the molecular lattice undergoing almost no deformation before and after gas adsorption, and the adsorption sites being limited to positions directly involved in mediating magnetic interactions. The findings of this research more clearly indicate that oxygen adsorption is directly involved in magnetic phase control in MOF magnets.
Fig. 1 Schematic diagram of a layered MOF magnet synthesized from an electron donor (a paddlewheel-type diruthenium center) and an acceptor (a TCNQ derivative)
Credit: Yasutaka Kitagawa
Fig. 2 Temperature dependence of magnetization before and after adsorbing carbon dioxide (external magnetic field 100 Oe). Before the introduction of carbon dioxide (in a dry state), the material is ferromagnetic with a magnetic phase transition temperature (Tc) of 30 K. After carbon dioxide adsorption, the Tc changes to 24 K, but the magnetic order remains ferromagnetic (inserted figure). The repeated adsorption and desorption of carbon dioxide make Tc change repeatedly.
Credit: Yasutaka Kitagawa
Fig. 3 Temperature dependence of magnetization in the oxygen-adsorbed state (external magnetic field of 100 Oe). In the inserted figure, the vertical axis is regulated to the magnetization value at 2 K as 1, to be able to see the antiferromagnetic phase transition easier. Before the introduction of oxygen (in a dry state), it is a ferromagnetic material with a magnetic phase transition temperature (Tc) of 30 K. In the oxygen-adsorbed state (sample adsorbed with oxygen for 12 hours at 160 K), a peak appears at 28 K, indicating that the sample has become an antiferromagnetic body with a magnetic phase transition temperature (TN) of 28 K. During that time, a peak indicating TN was observed at 17 K in the sample after 0.5 h (arrow in the inserted diagram), and as the amount of adsorbed oxygen increased (1 h, 2 h, 6 h), TN shifted toward higher temperatures.
Credit: Yasutaka Kitagawa
Fig. 4 Crystal structure of carbon dioxide adsorbed state and oxygen adsorbed state. Only the gas molecules adsorbed between the layers and their surrounding frameworks are indicated. Although the environment around the adsorbed gas molecules is almost the same in both cases, the magnetic phase change occurs only when oxygen is adsorbed, suggesting the importance of adsorbed oxygen.
Future Development
Oxygen is one of the smallest molecular units that possesses the property of electron spin, which is the origin of magnets. This study demonstrated that it is possible to design materials that utilize electron spin, paving the way for the realization of new molecular devices that selectively control oxygen. Furthermore, the MOF magnet identified in this study has great potential not only as a binary ON/OFF switch, but also as a material that outputs a continuous analog signal depending on the amount of adsorbed gas. The achievements serve as a guideline for designing next-generation smart porous magnets, both in terms of basic science and applied research aimed at developing high-performance molecular devices.
Notes
The article, “Cooperative Magnetic Phase Evolution via Oxygen Spin Coupling in a Layered Metal−Organic Framework,” was published in Journal of the American Chemical Society at DOI: https://pubs.acs.org/doi/10.1021/jacs.5c12038.
