Discovery of a new mechanism controlling natural killer T (NKT) cell differentiation
Expect for applications in cancer immunotherapy and infectious diseases
- The research group has discovered a new transcriptional control mechanism that regulates the differentiation of NKT cells, a type of innate-like T cells whose differentiation mechanism has long remained unknown.
- The researchers found that NKT cells disappeared in mice lacking the phosphorylation of protein kinase D (PKD) in T cells alone.
- It is found that PKD phosphorylates the transcription factor Ikaros, and that Ikaros induces the expression of PLZF, a transcription factor essential for innate-like T cell differentiation, thereby promoting NKT cell differentiation.
- Since NKT cells play an important role in eliminating cancer cells and infected cells, controlling the function of PKD is expected to be applied to the treatment of cancer immunity, infectious diseases, and autoimmune diseases in which NKT cells are involved in pathogenesis.
Outlines
A research group including Assistant Professor Eri Ishikawa of the Research Institute for Microbial Diseases, and Professor Sho Yamasaki, concurrently serving as members of Immunology Frontier Research Center, Center for Infectious Disease Education and Research , Vaccines Development Center, Center for Advanced Modalities and Drug Delivery System (DDS)at the University of Osaka has uncovered a new transcriptional control mechanism for natural killer T (NKT) cell differentiation.
NKT cells are a type of innate-like T cells that play an intermediate role between innate and adaptive immunity, and are known to be involved in various diseases, such as eliminating cancer cells and infected cells, and the pathogenesis of autoimmune diseases. The differentiation mechanism of these cells, which differentiate in the thymus, remains largely unknown compared to conventional T cells, which also differentiate in the thymus, and the full picture has not yet been clarified.
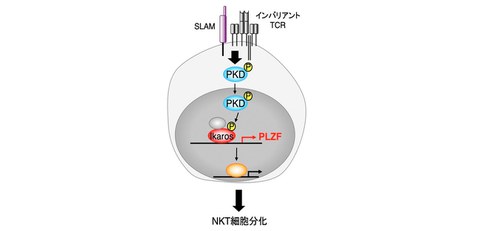
In this study, the research group found that NKT cells disappear in mice lacking PKD in T cells of the serine/threonine kinase, protein kinase D (PKD). These mice had reduced expression of promyelocytic leukemia zinc finger (PLZF), a transcription factor essential for the differentiation of innate-like T cells, demonstrating that PKD contributes to NKT cell differentiation through the induction of PLZF expression (Fig. 1). The researchers also identified the transcription factor Ikaros as a substrate of PKD in NKT cells and demonstrated that Ikaros activates the transcription of PLZF.
This suggests that controlling PKD activation may regulate the differentiation and supply of NKT cells, which could be applied to cancer immunotherapy and the treatment of autoimmune diseases.
Fig. 1 PKD contributes to NKT cell differentiation through induction of PLZF expression
Credit: Sho Yamasaki
Research Background
NKT cells, a type of innate-like T cells, differentiate in the thymus just like conventional T cells. However, while conventional T cells differentiate by recognizing self-peptides presented on MHC molecules using a highly diverse T cell receptor (TCR), NKT cells differentiate by recognizing lipids presented on CD1d molecules using a less diverse invariant TCR (iTCR). Although PLZF, whose expression is induced by signals mediated by iTCR, is known to be an essential transcription factor for the differentiation of innate-like T cells, the details of the molecular mechanism leading to PLZF expression from iTCR have not been elucidated.
Research Contents
The research group previously discovered that protein kinase D (PKD) is activated by TCR signals and that of the three similar isoforms encoded by different genes, PKD2 and PKD3 are expressed in T cells. Then, the research group established and analyzed mice lacking PKD2/3 specifically in T cells, and found that while conventional T cells remained normal, only NKT cells were almost completely eliminated (Fig. 2). When the small number of NKT cells present in PKD2/3-lacking mice was examined, the increase in PLZF expression was attenuated compared to wild-type mouse NKT cells. When introducing PLZF into deficient mice via a transgene, it is found that the number of NKT cells was restored (Fig. 3) and PKD acts to differentiate NKT cells through the induction of PLZF expression.
Fig. 2 NKT cells are lost in mice lacking PKD2/3 in T cells
Credit: Sho Yamasaki
Fig. 3 Increased PLZF expression was attenuated in PKD2/3-lacking NKT cells (top), and the number of NKT cells was restored by introducing PLZF (bottom)
Credit: Sho Yamasaki
Next, to clarify the molecular mechanism linking PKD to PLZF expression, the research group performed phosphorylation proteomics analysis using tandem mass tags (TMT) to search for PKD substrates. As a result, the researchers identified the transcription factor Ikaros as a candidate molecule (Fig. 4). Kinase assays clarified that Ikaros is directly phosphorylated by PKD, and luciferase reporter assay clarified that Ikaros activates PLZF transcription (Fig. 5). The research group also established and analyzed knock-in mice of the phosphorylation-defective mutant Ikaros, in which the serine residue phosphorylated by PKD was replaced with alanine, and found a decrease in NKT cells (Fig. 6). These results revealed the existence of the PKD-Ikaros-PLZF pathway as a new transcriptional control mechanism regulating NKT cell differentiation.
Fig. 4 Tandem mass tag (TMT)-based phosphorylation proteomics (left) identified candidate PKD substrates candidate, including Ikaros (right)
Credit: Sho Yamasaki
Fig. 5 Ikaros activated the transcription of PLZF
Credit: Sho Yamasaki
Fig. 6 NKT cells were reduced (right) in phosphorylation-defective mutant Ikaros knock-in mice (left)
Credit: Sho Yamasaki
Social Impact of the Research
This study revealed the existence of a new transcriptional control mechanism, the PKD-Ikaros-PLZF pathway, which links iTCR signals to PLZF expression and NKT cell differentiation. While NKT cells play a protective role against the onset and progression of various diseases, they are also known to have harmful effects. By controlling PKD activity, it may be possible to regulate the differentiation and supply of NKT cells, which is expected to be applied to the treatment or prevention of these diseases.
It has been revealed that the transcription factor Ikaros, which works in the nucleus, is phosphorylated by PKD. However, it is still unknown where in the cell Ikaros is phosphorylated, or how phosphorylation changes the localization of Ikaros and its activation as a transcription factor, leading to the induction of PLZF expression. Future research is expected to shed light on these issues.
In mice lacking PKD2/3 in T cells, innate-like T cells other than NKT cells were also reduced. By further verifying whether the transcriptional control mechanism of NKT cell differentiation revealed in this study is a mechanism common to innate-like T cells, it is possible to clarify the universal differentiation control mechanism of innate-like T cells.
Notes
The article, “Invariant TCR-triggered Protein kinase D activation mediates NKT cell development,” was published in American scientific journal of Journal of Experimental Medicine (Online) at DOI: https://doi.org/10.1084/jem.20250541.
