Can the mechanism that prevents cancer also be involved in sperm motility?
Mechanism by which lipids regulate voltage-sensing phosphatase (VSP) essential for sperm
- It is elucidated that the mechanism by which the function of voltage-sensing phosphatase (VSP), an enzyme responsible for sperm motility, is appropriately regulated by phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2).
- Although it has been clarified that VSP function is important for sperm motility control, it has remained unclear how this function is regulated. In this study, a combination of experiments using fluorescent dyes and simulations successfully demonstrated the interaction between PI(4,5)P₂ and the VSP linker.
- It is expected to contribute to the development of technologies to control the function of the tumor suppressor phosphatase and tensin homolog (PTEN), which has an enzymatic site similar to VSP, and to the development of treatments for male infertility through understanding sperm motility.
Outlines
A collaborative research group consisting of Specially Appointed Assistant Professor Natsuki Mizutani (Full Time) (at the time of the research) and Professor Yasushi Okamura (Integrated Physiology) of the Graduate School of Medicine, the University of Osaka, Professor Yasushige Yonezawa of the High Pressure Protein Research Center, Advanced Technology Research Institute, Kindai University, and Professor Atsushi Nakagawa Laboratory for Supramolecular Crystallography of the Institute for Protein Research of the University of Osaka has elucidated the mechanism by which the function of an enzyme molecule called voltage-sensing phosphatase (VSP), which is essential for sperm motility, is controlled by the phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2). This mechanism was found to be the same as the regulatory mechanism for the enzyme PTEN, which has the function of preventing cancer.
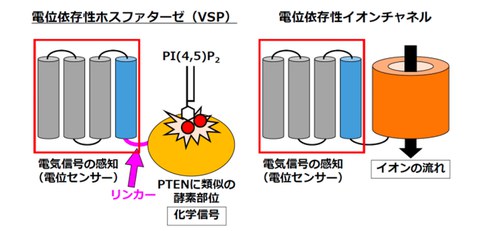
VSP is a molecule discovered by the research group in 2005, and it contains a voltage sensor domain (VSD) that detects changes in the cell membrane potential and an enzyme site similar to PTEN (Fig. 1). It has been clarified that the function of this enzyme is important in controlling sperm motility, but how this function is regulated has not been elucidated.
The research group investigated changes in the fluorescence intensity of a fluorescent unnatural amino acid, 3-(6-acetylnaphthalen-2-ylamino)-2-aminopropanoic acid (Anap) incorporated into VSP, and revealed that PI(4,5)P₂ interacts with the linker that connects the VSD and the enzyme site (Fig. 2A). Combined with the results of molecular dynamics simulations, the researchers elucidated that this interaction controls the function of the VSP enzyme. This result is expected to lead to a better understanding of the mechanism that regulates PTEN enzymatic activity and the sperm motility, which are still largely unknown.
Fig. 1 Structure and function of VSP
VSPs consist of a voltage sensor common to voltage-gated ion channels (VGICs) and an enzymatic site similar to PTEN, and exhibit enzymatic activity that degrades the phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2)
Credit: Yasushi Okamura
Research Background
It has been known that in all living organisms, electrical signals (changes in the membrane potential), which are the source of complex and advanced biological phenomena, are sensed by protein molecules called voltage-gated ion channels (VGICs) and converted into ion flow (Fig. 1). Meanwhile, the VSP discovered by the research group is a completely new protein molecule that converts electrical signals into the action of an enzyme similar to PTEN, and it has been revealed by using mice that it plays an important role in controlling sperm dynamics.
Protein defect leaves sperm chasing their tails
Shocking revelation: an electrically-activated protein regulates spermatogenesis
The research group has previously reported that such intriguing functions are generated by direct interaction between a voltage sensor domain (VSD) and the enzyme site.
The mechanism of the VSP, a protein which is essential for sperm motility, is finally elucidated! (Japanese only)
However, the mechanism by which VSP function is properly controlled remains unclear.
Research Contents
The research group utilized the property of Anap, whose fluorescence intensity changes when it interacts with PI(4,5)P₂, and discovered that PI(4,5)P₂ interacts with the linker that connects the voltage sensor domain (VSD) and the enzyme site. This change in fluorescence intensity was also observed in mutants that could eliminate the influence of the substrate PI(4,5)P₂, which is decomposed by VSP, revealing an interaction between the linker and a PI(4,5)P₂ molecule other than the substrate. It was also reproduced by molecular dynamics simulations. Since the electrical signal was not converted into a chemical signal when no interaction occurred, it is clarified that PI(4,5)P₂ controls the function of VSP (Fig. 2A). These findings suggest that PI(4,5)P₂ regulates sperm VSP function within an appropriate range, thereby controlling sperm motility. On the other hand, PI(4,5)P₂ is known to regulate the function of PTEN through a similar interaction, which is thought to suppress cancer (excessive cell proliferation) (Fig. 2B).
Fig. 2 Regulatory mechanism of VSP and PTEN by PI(4,5)P₂
A. PI(4,5)P₂ interacts with the linker between the voltage sensor domain (VSD) and the enzyme site, regulating the function of VSP, which is thought to control sperm motility
B. A similar interaction is also seen in the regulation of PTEN function, which is thought to lead to the suppression of carcinogenesis
Credit: Yasushi Okamura
Social Impact of the Research
The results of this research will advance the understanding of the mechanism of PTEN enzyme activity control and sperm dynamics, and are expected to develop cancer and male infertility treatments through PTEN control in the future. Furthermore, because voltage-gated ion channels (VGICs), proteins associated with various neurological and cardiac diseases, share voltage sensors with VSPs, this research is an important achievement in understanding the mechanism by which electrical signals are converted into ion flow.
Notes
The article, “Nonsubstrate PI(4,5)P₂ interacts with the interdomain linker to control electrochemical coupling in voltage-sensing phosphatase (VSP),” was published in American scientific journal of Proceedings of the National Academy of Sciences of the United States of America (PNAS) (Online) at DOI: https://doi.org/10.1073/pnas.2500651122
