The world's first! Creating a new group of layered materials using platinum oxide
Computationally assisted innovation in high-pressure materials development
- The world's first successful synthesis of the layered homologous series Na(PtO₂)2n+1 (n=1, 2) platinum oxide with rutile-type structure as the layer matrix.
- The development of platinum oxides has been delayed due to its chemical inactivity and strong reduction tendency, but this problem has been overcome by utilizing a highly oxidizing atmosphere created by a high-pressure synthesis method.
- By effectively combining solid-state chemistry knowledge with stability predictions based on first-principles calculations, development of a new group of layered materials has been succeeded.
Outlines
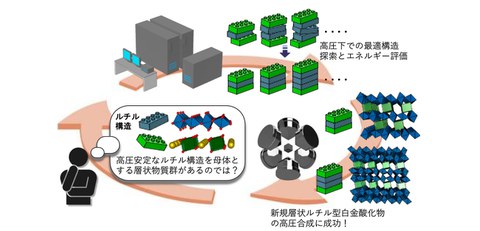
A research group including Yasuhito Kobayashi (doctor course), Associate Professor Hidefumi Takahashi, and Professor Shintaro Ishiwata of the Graduate School of Engineering Science at the University of Osaka, in collaboration with Assistant Professor Shunsuke Kitou of the Graduate School of Frontier Sciences at the University of Tokyo, has succeeded in synthesizing the world's first homologous series of layered platinum oxides, Na(PtO₂)2n+1 (n=1, 2), with a rutile-type structure as the layer matrix, and identifying their structure. This result has been achieved by combining structural stability predictions made by first-principles calculations based on knowledge of oxide structures with ultra-high-pressure synthesis method (Fig. 1).
Platinum oxide has been widely studied as a functional material, and is utilized as a catalyst material to promote reduction reactions in a hydrogen atmosphere. On the other hand, as platinum is chemically stable and has low reactivity, it has been difficult to develop platinum oxides with new structures, and no homologous series layered materials have been known until now. In this study, by utilizing a high-pressure synthesis method accompanied by an ultra-high oxidizing atmosphere, the researchers succeeded in synthesizing single crystals of a novel layered oxide, NaPt₃O₆, with a two-dimensional rutile-type structure as the layer matrix. Furthermore, they designed materials focusing on the fact that the rutile structure is a dense structure that is advantageous for stability under pressure and byusing first-principles calculations they predicted the stability of NaPt₅O₁₀, a highly layered structure, and succeeded in synthesizing its single crystal under high pressure. This demonstrates that the complementary collaboration between computational science and solid-state chemistry is extremely effective in accelerating the development of new materials through high-pressure synthesis. In new materials discovered in this study, PtO₆ octahedra form two-dimensional rutile-type layers, with PtO planes forming one-dimensional chains between the layers. This is an unprecedented new homologous series of oxides, defined by the general formula Na(PtO₂)2n+1. This study not only provides a novel structure, but also a new platform for controlling the dimensionality of platinum oxides and exploring their physical properties. Furthermore, this approach is an effective strategy that enables the systematic exploration of unknown metastable phases, and the results provide an effective guideline for the development of new materials.
Fig. 1 Discovery of a new group of layered materials through effective collaboration between solid-state chemical knowledge and first-principles calculations
Research Background
Transition metal oxides are a group of materials that exhibit a variety of functions, including high-temperature superconductivity, battery materials, and catalysts. The typical example is the layered homologous series Ruddlesden-Popper phase, which has a perovskite-type structure as the layer matrix in 3d transition metal oxides. On the other hand, late 5d transition metals, including platinum, are noble metals which have a high chemical inactivity and tendency to reduce, making them incompatible with oxides and making material discovery difficult. However, platinum is an element with the unique feature of being able to form not only the octahedral coordination with oxygen that is characteristic of transition metals, but also a square-planar coordination, and is thought to potentially have a great diversity of crystal structures.
Research Contents
Professor Ishiwata and his research group have succeeded in synthesizing a single crystal of a new oxide, NaPt₃O₆, composed of sodium (Na) and platinum (Pt), by utilizing a high-pressure synthesis method that enables an ultra-high oxidizing atmosphere. X-ray diffraction (SXRD) experiments using single crystals at the large synchrotron radiation facility SPring-8 (BL02B1) revealed that the PtO₆ octahedra form two-dimensional layers with a rutile-type structure, and that the square-planar PtO₄ overlap between the layers to form one-dimensional chains, creating a unique structure. A typical example of a layered homologous series oxide obtained by high-pressure synthesis method is the Ruddlesden-Popper phase, which has a perovskite-type structure as the layer matrix. Its general formula is An+1BnO₃n+1, where n is the number of perovskite layers. On the other hand, although the rutile-type structure has a dense structure that is easily stabilized under pressure, similar to the perovskite-type structure, no layered homologous series using this as the layer matrix had been discovered. In this study, the research group focused on the fact that the new material NaPt₃O₆ is a layered material containing a rutile-type structure and designed a high-order layered structure with the number of rutile-type layers, n, of two or more, and by evaluating the energy of the optimized structure under high pressure using first-principles calculations, it is predicted that NaPt₅O₁₀ with n=2 could be obtained through high-pressure synthesis. Based on these calculation results, the research group attempted a high-pressure synthesis method and performed X-ray diffraction (SXRD) experiments on the obtained single crystals, revealing that the crystal structure was the same as that obtained from the first-principles calculations (Fig. 2). Therefore, it was revealed that these new materials are a layered homologous series expressed by the general formula Na(PtO₂)2n+1 (n=1, 2) (n=∞ is rutile-type PtO₂ known as a catalytic material). It is expected that various rutile-type layered oxides, including those with n=3 or more, will be discovered in the future.
Fig. 2 Crystal structure of Na(PtO₂)2n+1 (left) and energy phase diagram calculated by first-principles calculations (right)
Social Impact of the Research
The results of this research are expected to provide a new group of functional oxides based on a new structural motif called "rutile," in addition to the group of functional oxides that have been pursued in the homologous series based on the conventional perovskite-type structure. In the future, systematic research into magnetic, conductive, and catalytic properties is expected, and applications to spintronics and oxygen reduction catalysts are also within reach. Furthermore, this research demonstrates that using first-principles calculations as the starting point for material exploration is an effective strategy that enables the systematic exploration of unknown metastable phases, and can be an outcome that provides new guidelines for materials science.
Notes
The article, “A rutile-based homologous series Na(PtO₂)2n+1 discovered by computationally assisted high-pressure synthesis,” was published in British Journal of Inorganic Chemistry as a Featured Article at DOI: https://doi.org/10.1021/acs.inorgchem.5c02074. It has also been selected as the ACS Editors' Choice.


