New organic molecule shatters phosphorescence efficiency records and paves way for rare metal-free applications
A research team led by Osaka University discovered that the new organic molecule thienyl diketone shows high-efficiency phosphorescence. It achieved phosphorescence that is more than ten times faster than traditional materials, allowing the team to elucidate this mechanism.
Phosphorescence is a valuable optical function used in applications such as organic EL displays (OLEDs) and cancer diagnostics. Until now, achieving high-efficiency phosphorescence without using rare metals such as iridium and platinum has been a significant challenge. Phosphorescence, which occurs when a molecule transitions from a high-energy state to a low-energy state, often competes with non-radiative processes where the molecule loses energy as heat.
This competition can lead to slow phosphorescence and lower efficiency. While previous research indicated that incorporating certain structural elements into organic molecules could speed up phosphorescence, these efforts have not matched the speed and efficiency of rare metal-based materials.
The research team’s breakthrough with the new organic molecule thienyl diketone represents a significant advancement in the field. Yosuke Tani, senior author of the study, remarked, "We discovered this molecule by chance and initially did not understand why it demonstrated such superior performance. However, as our research progressed, we began to connect the pieces and deepen our understanding."
"Our research has led to a clearer understanding of the mechanism behind this molecule’s performance than any previous organic phosphorescent material,” explains Dr. Tani. “Nonetheless, we believe there is still much to explore, and we are excited about its potential applications."
This research provides new design guidelines for developing organic phosphorescent materials that do not rely on rare metals, offering the potential to surpass and replace these materials in various applications. The findings promise significant advancements in the fields of OLEDs, lighting, and medical diagnostics, among others.
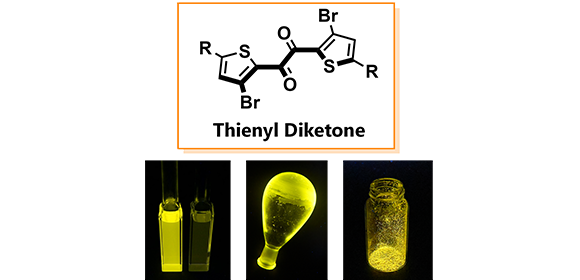
Fig. 1
Chemical structure of the molecule and photographs of phosphorescence taken under UV irradiation.
Credit: Osaka University
Fig. 2
Graph showing the acceleration of phosphorescence and its impact on the efficiency. Orange diamonds are thienyl diketones and blue dots are previous molecules. kp represents the phosphorescence rate. Increase of kp enables the improve of efficiency.
Credit: Osaka University
Fig. 3
Picture featuring the mechanism of fast phosphorescence. The blue light rays come together into the molecule to generate bold yellow pillars, representing the acceleration of phosphorescence through the mixing of singlet states.
Credit: Copyright © 2024 YAP. Co., Ltd.
The article, “Fast, efficient, narrowband room-temperature phosphorescence from metal-free 1,2-diketones: rational design and mechanism,” was published in Chemical Science at DOI: https://doi.org/10.1039/D4SC02841D.
Related Links
Assistant Professor TANI Yousuke (Researcher Directory)
Assistant Professor TANI Yousuke (researchmap)
Kubo Laboratory, Department of Chemistry, Graduate School of Science, Osaka University
Associate Professor MIYATA Kiyoshi (researchmap)
Laboratory of Spectrochemistry, Kyushu University
EurekAlert!
Asia Research News
