Pulling out the stops: Deletion of regnase-1 promotes anti-tumor activity in NK cells
Natural killer (NK) cells play a crucial role in the first line of host defense by eliminating bacteria, viruses, and mutated cells that might become cancer cells. While the interferon-gamma (IFN-γ) typically plays a pivotal role in the function of NK cells, the detailed mechanisms of its regulation have not been fully elucidated. In clinical cancer treatment, particularly in anti-tumor immunotherapy, the challenge lies in regulating the effective infiltration of NK cells and T cells into tumor tissues, activating them, and retaining them within the tumor for more effective cancer therapy.
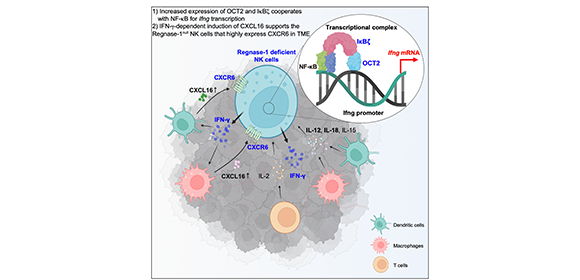
Performing single-cell RNA sequencing (scRNA-seq) analysis of tumor cells from wild-type mice and NK cell-specific Regnase-1 deficient mice, a research collaboration led by Xin Sun, Diego Diez, and Shizuo Akira at the Immunology Frontier Research Center (IFReC) of Osaka University and Yasuharu Nagahama at the Osaka Research Center for Drug Discovery of Otsuka Pharmaceutical Co., Ltd. has revealed that deletion of mRNA endonuclease Regnase-1 promoted NK cell anti-tumor activity via OCT2-dependent transcriptional up-regulation of Ifng mRNA. Their findings are illustrated in Figure 1. Dr. Nagahama, a lead co-author of the study, explained, “Our investigation showed the contribution of B cell-specific OCT2 transcription factor for NK cell biology, particularly in the transcriptional regulation of Ifng." Additionally, the study found that Regnase-1 deficiency induces high expression of CXCR6 in NK cells, a chemokine receptor of which expression is typically lost in mature murine NK cells. Furthermore, IFN-γ-mediated induction of CXCL16 expression in myeloid cells supports NK cell infiltration and persistence in the tumor microenvironment via the CXCR6-CXCL16 axis.
In current cancer immunotherapy, immune checkpoint inhibitors such as anti-PD-1 and anti-CTLA-4 antibodies have demonstrated significant therapeutic efficacy against leukemias and lymphomas. However, unlike traditional chemotherapies and kinase inhibitors, the results yielded by anti-tumor immunotherapy for solid tumors have not been as promising. The findings of this study potentially overcome the challenges of immune checkpoint inhibitors against solid tumors by promoting infiltration and persistence of immune cells that have cytotoxic activity, such as NK cells and T cells, within the tumor microenvironment. This approach augments the production of cytotoxic proteins such as IFN-γ, Granzymes, and Perforin from these immune cells. Additionally, targeting the expression or function of Regnase-1 in these immune cells sheds light on the development of a promising strategy for anti-tumor immunotherapy. Furthermore, combining the loss-of-function strategy targeting Regnase-1 with current anti-tumor immunotherapies, such as immune checkpoint inhibitors and CAR-T cell therapy, has the potential to synergistically enhance therapeutic efficacy against solid tumors.
Fig. 1
Regnase-1 deficient NK cells highly express Ifng depend on the cooperation of OCT2, IκBζ, and NF-κB transcriptional complex. This transcriptional upregulation of Ifng induced the high IFN-γ production from NK cells, leading to the alteration of tumor microenvironment that support the activation and persistence of NK cells inside the tumor microenvironment.
Credit: Yasuharu Nagahama (Created with BioRender.com)
The article, “Deletion of the mRNA endonuclease Regnase-1 promotes NK cell anti-tumor activity via OCT2-dependent transcription of Ifng” was published in Immunity at DOI: https://doi.org/10.1016/j.immuni.2024.05.006.
