No cap removal, no fertilization
Researchers from Osaka University have identified a protein known as FER1L5, a member of the ferlin protein family, that is essential for male fertility in mice
Fertilization is the union of two cells: an egg and a sperm. Before the egg and the sperm fuse, an event known as the “acrosome reaction” needs to occur in the sperm. Now, a team from Osaka University has identified a protein called FER1L5 that is essential for sperm to undergo the acrosome reaction.
There is a cap-like vesicle structure called the acrosome over the front of the head of a sperm. As the sperm migrates in the female reproductive tract in mammals, the acrosome reaction occurs, which involves the release of molecules in the acrosome to facilitate fertilization. Although the acrosome reaction is essential for sperm to fertilize eggs, the molecular mechanism that regulates the acrosome reaction remains unclear.
The research team examined FER-1, a ferlin family protein essential for fertilization in the nematode (i.e., roundworm) called C. elegans. Unlike mammalian sperm, such as those of humans and mice, C. elegans sperm move like amoebae. FER-1 has been found to be important for membranous organelle fusion and the initiation of amoeboid movement. In mice, six ferlin family proteins are similar to FER-1: DYSF, OTOF, MYOF, FER1L4, FER1L5, and FER1L6. While DYSF is known to be involved in muscular dystrophy and OTOF in deafness, clarification is needed on whether proteins similar to FER-1 are involved in mammalian sperm function.
“When we generated mice that were lacking one particular member of the ferlin family, FER1L5, the sperm of the male mice were unable to fertilize eggs,” explains first author Akane Morohoshi. However, the fertility of female mice lacking FER1L5 was unchanged. The team then went on to show that sperm lacking FER1L5 were unable to undergo the acrosome reaction. “Even though we added a strong inducer, no acrosome reaction took place in sperm lacking FER1L5, suggesting that FER1L5 is critical for the acrosome reaction,” explains senior author Haruhiko Miyata.
“Our results indicate that the function of ferlin proteins is well conserved from nematodes to mice, although their sperm look different,” explains senior author Masahito Ikawa. While this study was carried out on mice, FER1L5 protein is known to be present in human sperm. Further research resulting from this study may therefore lead to new treatments and diagnostic methods for male infertility in humans.
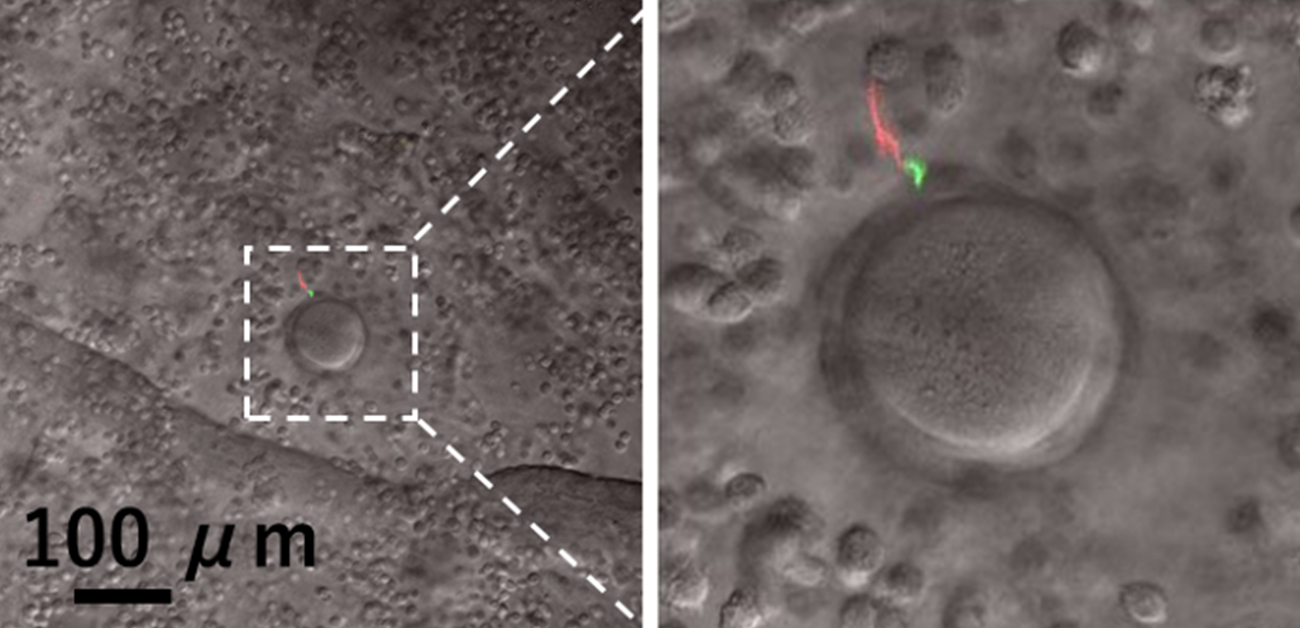
Fig. 1
Sperm (red) reaching an egg in the female reproductive tract. Sperm lacking FER1L5 cannot undergo the acrosome reaction, and the acrosome (green) remains when it reaches the egg. Therefore, sperm are unable to fertilize eggs.
Credit: © 2023, Masahito Ikawa, © 2023 Morohoshi et al., Science Advances
Fig. 2
Changes to the sperm. C. elegans sperm are activated and exhibit amoeboid movement. Mouse sperm undergo the acrosome reaction, exposing molecules essential for fusion with eggs. MO: membranous organelle.
Credit: © 2023, Masahito Ikawa, © 2023 Morohoshi et al., Science Advances
Fig. 3
Electron microscopic observation of sperm heads. Even with the addition of a strong inducer, the acrosome reaction (AR) does not occur in sperm lacking FER1L5, leaving the acrosome intact.
Credit: © 2023, Masahito Ikawa, © 2023 Morohoshi et al., Science Advances
The article, “Testis-enriched ferlin, FER1L5, is required for Ca2+-activated acrosome reaction and male fertility”, was published in Science Advances at DOI: https://doi.org/10.1126/sciadv.ade7607.
