Testing embryos for genetic conditions – Who is eligible and how are decisions made?
Researchers from Osaka University investigate the various approaches to determining which genetic conditions are eligible and which individual cases should be approved for preimplantation genetic testing
With advances in medical technology, embryos can be genetically screened before being implanted in the womb during IVF. Couples at risk for certain genetic conditions can now safely conceive children who will not inherit these conditions. However, establishing when such treatment is justified raises complex questions.
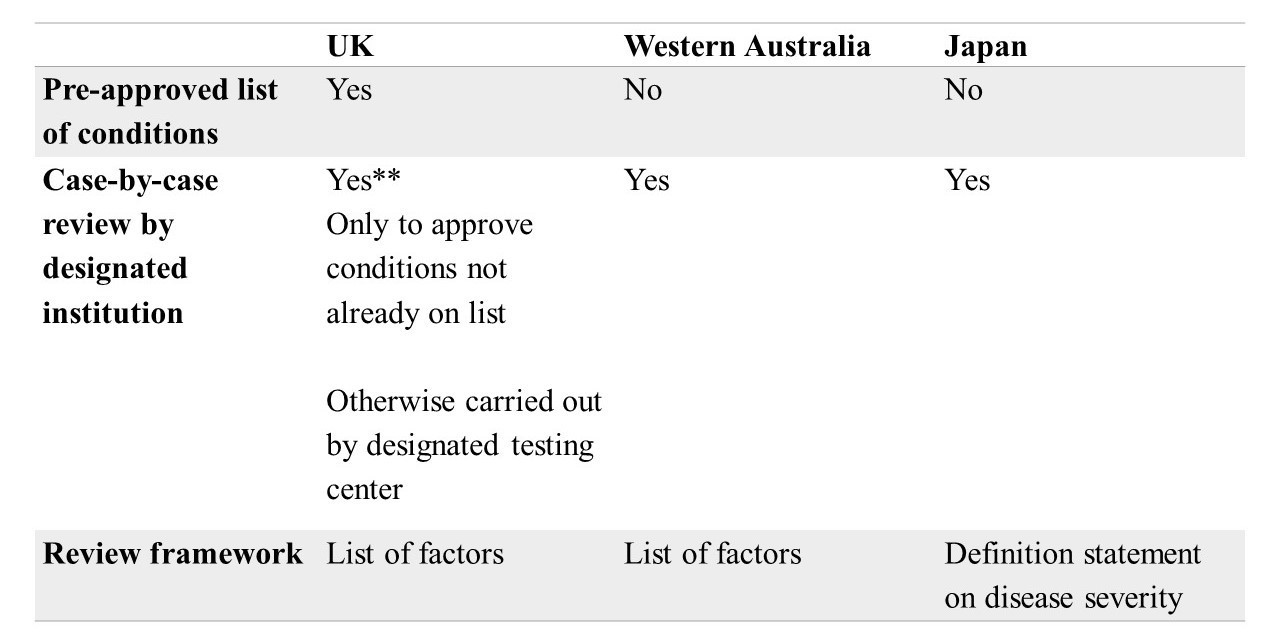
In a study published in Human Genomics, researchers from Osaka University conducted case studies of the regulatory frameworks governing preimplantation genetic testing for single-gene diseases (PGT-M) in Japan, the United Kingdom, and Western Australia.
While there is a general consensus that PGT-M should be used only in cases of a risk to the child of inheriting a “serious” genetic condition, this study is the first to describe in detail the criteria and factors that define disease severity when considering cases for PGT-M, along with how decisions are made when reviewing cases based on these criteria and factors.
“The question of what represents a ‘serious’ condition in the context of PGT is tricky,” comments lead author of the study Kate Nakasato. “A few years ago, there was a case in Japan of a patient with retinoblastoma – a genetically inherited cancer of the retina – who wished to conceive using PGT-M. While the case did not meet the standard for disease severity at the time, there were arguments from some who pointed out the potential impact on the daily life of the child. This ultimately resulted in a revision of Japan’s definition of disease severity for PGT-M, but it was not without debate about whether this would open the door to allowing PGT-M for other non-life-threatening conditions.”
One major difference among the three case studies was that the United Kingdom is unique in maintaining a list of over 600 genetic conditions that are pre-approved for PGT. This is not done in Japan or Western Australia, where each application is considered on a case-by-case basis.
“A concern about publishing such a list is that it has the potential to ‘label’ a genetic condition as medically undesirable,” notes Kazuto Kato, senior author. “As a result, the parents of children living with this condition – and possibly the children themselves – may feel the need to justify their birth. This can also affect how such individuals are treated in society.”
It is clear that PGT-M represents an extraordinary advance in reproductive medicine. However, as this study shows, how decisions regarding this treatment are made has social and ethical implications beyond any individual case. A multidisciplinary approach, drawing on individuals with the genetic conditions in question, as well as experts in disciplines such as the humanities, is necessary to meet this challenge.
Fig.1 Evaluation Methods
Licensed content. Nakasato K. et al. Evaluating standards for ‘serious’ disease for preimplantation genetic testing: a multi-case study on regulatory frameworks in Japan, the UK, and Western Australia. Human Genomics.
Credit must be given to the creator.
The article, “Evaluating standards for ‘serious’ disease for preimplantation genetic testing: A multi-case study on regulatory frameworks in Japan, the UK, and Western Australia,” was published in Human Genomics at DOI: https://doi.org/10.1186/s40246-022-00390-3.
