It's not all-right: problems when your body doesn't recognize your own left-handed RNA
Researchers from Osaka University use a mouse model to characterize a crucial enzyme’s inflammatory disease-relevant Z-RNA-binding ability
Like DNA, RNA molecules contain information through unique combinations of four different nucleotides. However, through a molecular process called RNA editing, chemical changes can be made to adenosine nucleotides that convert them to a nucleotide called inosine by enzymes known as adenosine deaminases (ADARs). ADAR-mediated RNA modification is essential for survival and two ADARs, ADAR1 and ADAR2, have been identified in mammals. In a recently published article in Immunity, a group at Osaka University studied mice containing specific mutations in ADAR1 and found that defects in the mutant enzyme RNA binding led to abnormal growth and development in the mice.
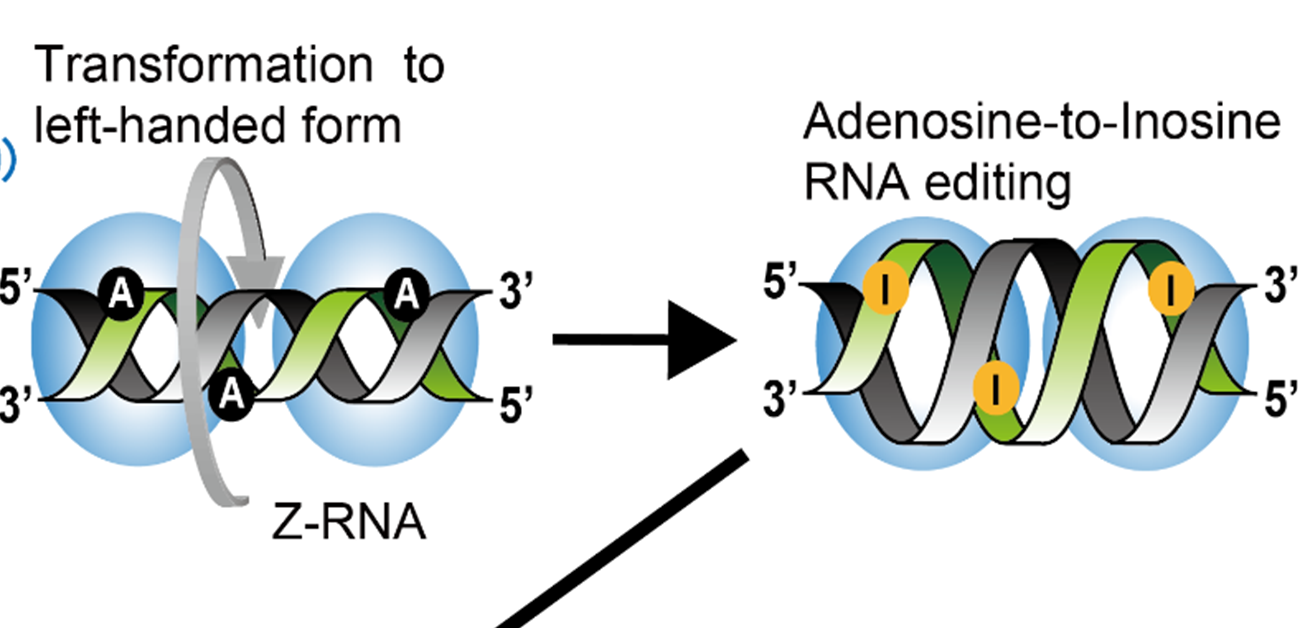
Two versions of ADAR1 protein exist in mouse cells: p110 and p150. Previous research suggested that ADAR1 edits double-stranded RNA (dsRNA) so that a cellular sensor called MDA5 recognizes it correctly as “self” RNA and does not mistake it for viral RNA (which would lead MDA5 to induce an immune response). Interestingly, the ADAR1 p150 enzyme contains a specific binding domain for a special type of RNA called Z-RNA. Double-stranded RNA typically forms a right-handed helical structure but Z-RNA is dsRNA that forms a left-handed structure.
“Mutations in ADAR1 p150, including within the domain that recognizes Z-RNA, have been associated with a genetic inflammatory disorder known as Aicardi-Goutières syndrome (AGS),” says lead author of the study Taisuke Nakahama. “We wanted to examine how this biological function affects AGS pathogenesis.”
To investigate this, the team generated genetically altered lab mice that had a point mutation in both alleles of the gene encoding ADAR1 p150. This mutation abolished the Z-RNA binding ability of the mutant ADAR1. Mutant mice showed severely inhibited growth relative to wild-type (non-mutant) mice.
“Mutant mice had abnormally developed organs, including critical ones like the brain, spleen, and colon,” explains senior author Yukio Kawahara. “Fascinatingly, their malformed brains showed characteristics similar to those of encephalopathy observed in human AGS patients.”
Mutant mice also displayed high expression levels of interferon-stimulated genes, resulting in a chronic inflammatory state. Through additional mechanistic experiments, the team demonstrated that the ADAR1 p150 Z-binding domain is essential for proper RNA editing catalyzed by this enzyme.
“Our work suggests that this domain’s interaction with Z-RNA is a critical initial step for preventing the immune system from believing this molecule is a foreign invader,” explains Nakahama.
This study pinpoints improper Z-RNA recognition as a contributor to AGS pathogenesis. These findings will assist in the development of novel therapeutic methods for treating this disorder and may also help us further understand responses to infections of RNA viruses like SARS-CoV-2.
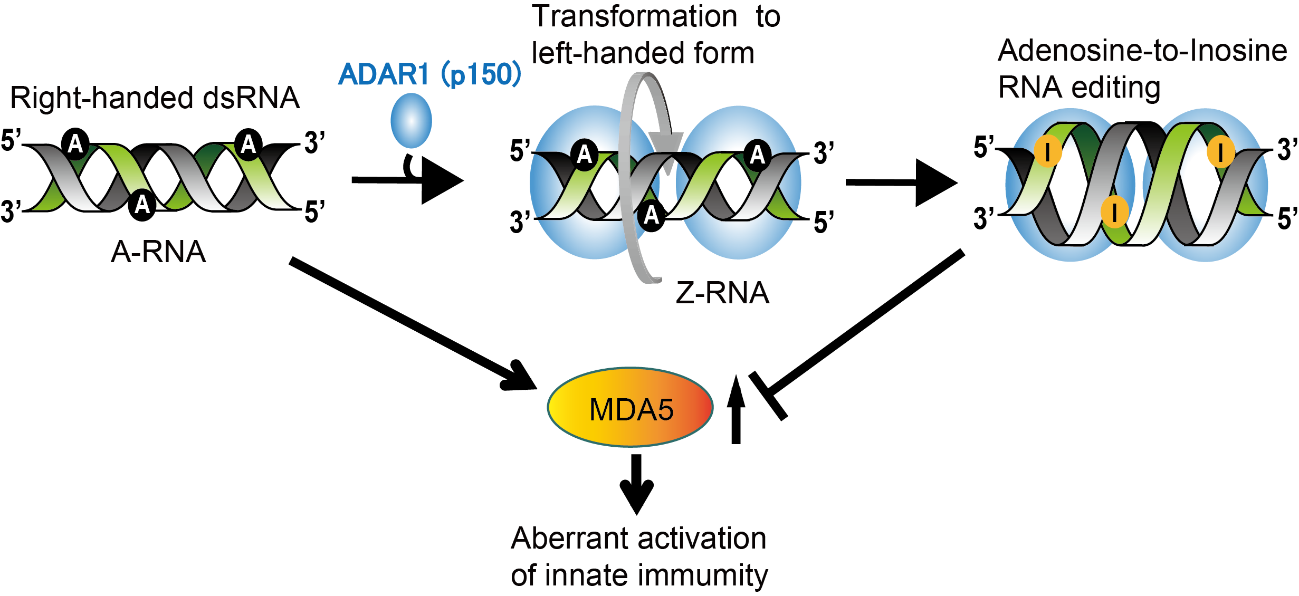
Fig.1 Adenosine-to-Inosine RNA editing in double-stranded (dsRNA) mediated by ADAR1 p150 prevents MDA5 activation that can lead to aberrant activation of innate immunity. During this editing process, right-handed dsRNA is transformed to left-handed (Z-form) dsRNA, which is mediated by the Za domain of ADAR1 p150. This is required for maintaining this enzyme’s proper RNA editing function.
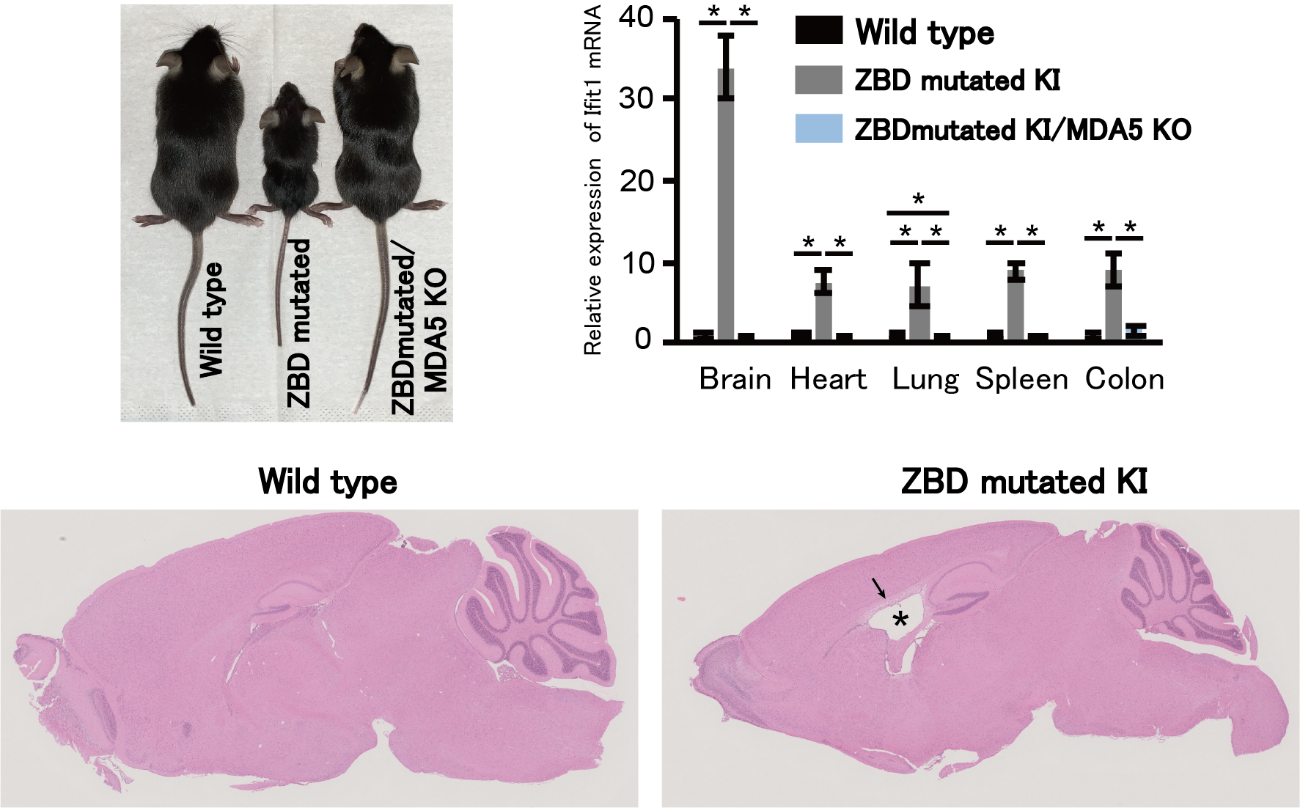
Fig.2 ADAR1 p150 Z-RNA binding domain (ZBD) mutated knock-in (KI) mice display severe growth retardation accompanied by Aicardi–Goutières syndrome (AGS)-like encephalopathy. Representative images of hematoxylin and eosin staining of brains show an enlarged lateral ventricle (*) and white matter vacuolation (arrow) in ZBD-mutated KI mice. The expression levels of interferon-stimulated genes (Ifit1 mRNA is shown as a representative gene) are increased, especially in the brain, in mutant mice. These abnormalities were ameliorated by concurrent deletion of MDA5 (MDA5 KO).
The article, “Mutations in the adenosine deaminase ADAR1 that prevent endogenous Z-RNA binding induce Aicardi-Goutières-syndrome-like encephalopathy,” was published in Immunity at DOI: https://doi.org/10.1016/j.immuni.2021.08.022.
