A winning combination for glycoprotein synthesis
Researchers from Osaka University report a fast, efficient method for synthesizing glycoproteins
Many processes in the body are regulated by the functions of proteins. For example, almost all molecules—such as DNA, proteins, oligosaccharides, and small bioactive molecules—are generated by enzymes. However, changes in protein functions in response to abnormal conditions cause critical diseases. Researchers from Osaka University have demonstrated a rapid, robust chemical method for preparing the highly pure (homogeneous) glycoproteins needed to investigate these changes. Their findings were published in the Journal of the American Chemical Society.
The efficacy of enzymes and functional proteins is regulated by protein modifications. A typical protein modification is glycosylation—adding sugar chains called glycans to proteins to give glycoproteins. Glycoproteins are found on the cell surface and in body fluids and play important roles in many biological processes. However, the glycoproteins formed can have many different glycan structures. Therefore, studying which glycan structures are essential for individual biological events is challenging.
The production of glycoproteins such as biologics—therapeutics produced from, or containing components of, living organisms—uses mammalian cell expression methods, but it is not possible to regulate the structure of the glycan added to the protein. Chemical synthesis is therefore the best way to make homogeneous glycoproteins that are appropriate for basic biological experiments. However, chemical methods require over 100 chemical conversion steps and are time-consuming.
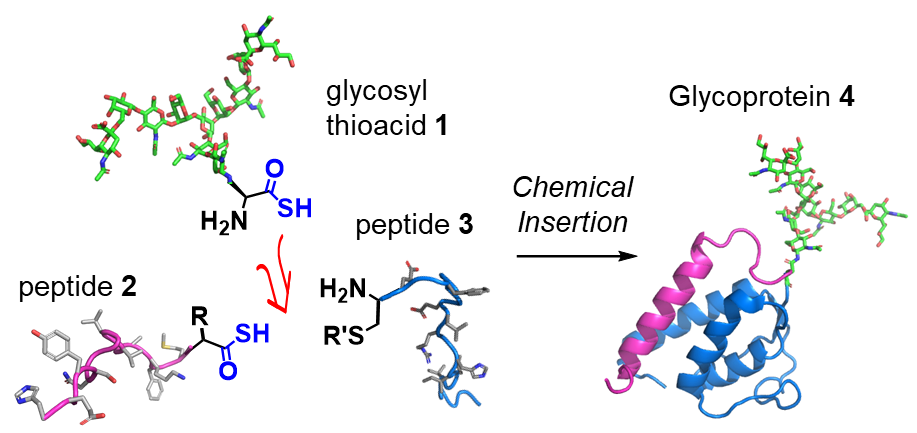
The Osaka researchers identified an unprecedented and efficient amide bond formation reaction between glycan-amino acid and two peptides: diacyl disulfide coupling and thioacid capture ligation. They demonstrated that glycosyl asparagine thioacid exhibited excellent chemoselective coupling with peptides, and the conditions used could generate glycosyl polypeptide within a few chemical conversion steps.
“We essentially used the glycosyl asparagine to form a junction between two functional peptides, giving a glycoprotein,” explains study first author Kota Nomura. “We achieved this in just a few steps, making it a highly efficient approach with little waste of valuable glycan materials.”
The team demonstrated the feasibility of their technique by synthesizing two cytokine glycoproteins. Cytokines are important bioactive molecules that are involved in inflammation and immune responses. Reliably producing them therefore provided important proof of the utility of the new synthetic route.
“We have demonstrated a reliable means of synthesizing glycoproteins that will allow the thorough study of glycan biological function, as well as the generation of biologics,” study corresponding author Yasuhiro Kajihara explains.
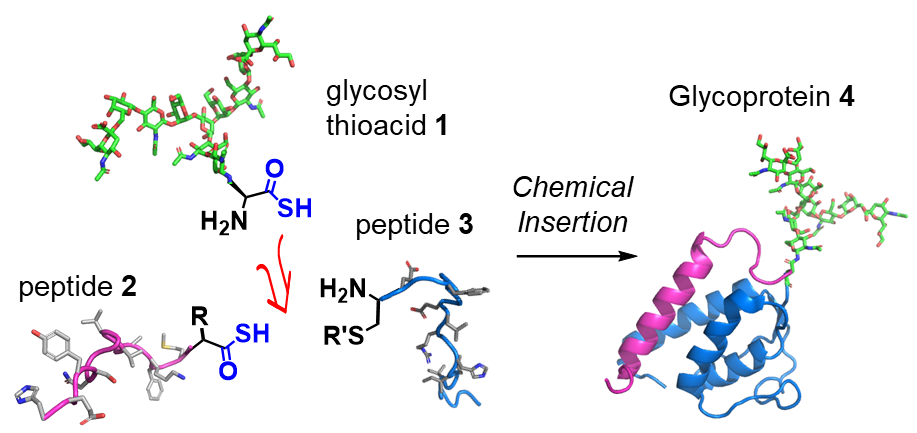
Glycoprotein synthesis by chemical insertion using a thioacid-mediated strategy: the method assembles a full glycoprotein segment in two steps.
The article, “Glycoprotein semisynthesis by chemical insertion of glycosyl asparagine using a bifunctional thioacid-mediated strategy,” was published in the Journal of the American Chemical Society at DOI: https://doi.org/10.1021/jacs.1c02601.
Related Links
Kajihara Yasuhiro (Researchers Database)
EurekAlert!
