Alpha-ray missile therapy: tumor cells attacked from intracellular region
Researchers at Osaka University have developed a technique of attacking cancer cells with lethal alpha rays from within by using a nutrient transporter to deliver radionuclides into malignant tumors
A cancer-specific L-type amino acid transporter 1 (LAT1) is highly expressed in caner tissues. Inhibiting the function of LAT1 has been known to have anti-tumor effects, but there has been limited progress in the development of radionuclide therapy agents targeting LAT1. Now, a multidisciplinary research team at Osaka University has established a targeted alpha-therapy with a novel drug targeting LAT1.
The researchers first produced the alpha-ray emitter 211Astatine, no easy task given that Astatine (At) is the rarest naturally occurring element on Earth. Targeted alpha-therapy selectively delivers α-emitters to tumors; the advantage over conventional β-therapy is that alpha decay is highly targeted and the high linear energy transfer causes double-strand breaks to DNA, effectively causing cell death. The short half-life and limited tissue penetration of alpha radiation ensures high therapeutic effects with few side-effects to surrounding normal cells.
Next, to carry the radioisotope into cancer cells, the researchers attached it to α-Methyl-L-tyrosine, which has high affinity for LAT1. This subterfuge exploits the elevated nutrient requirements of rapidly multiplying cancer cells.
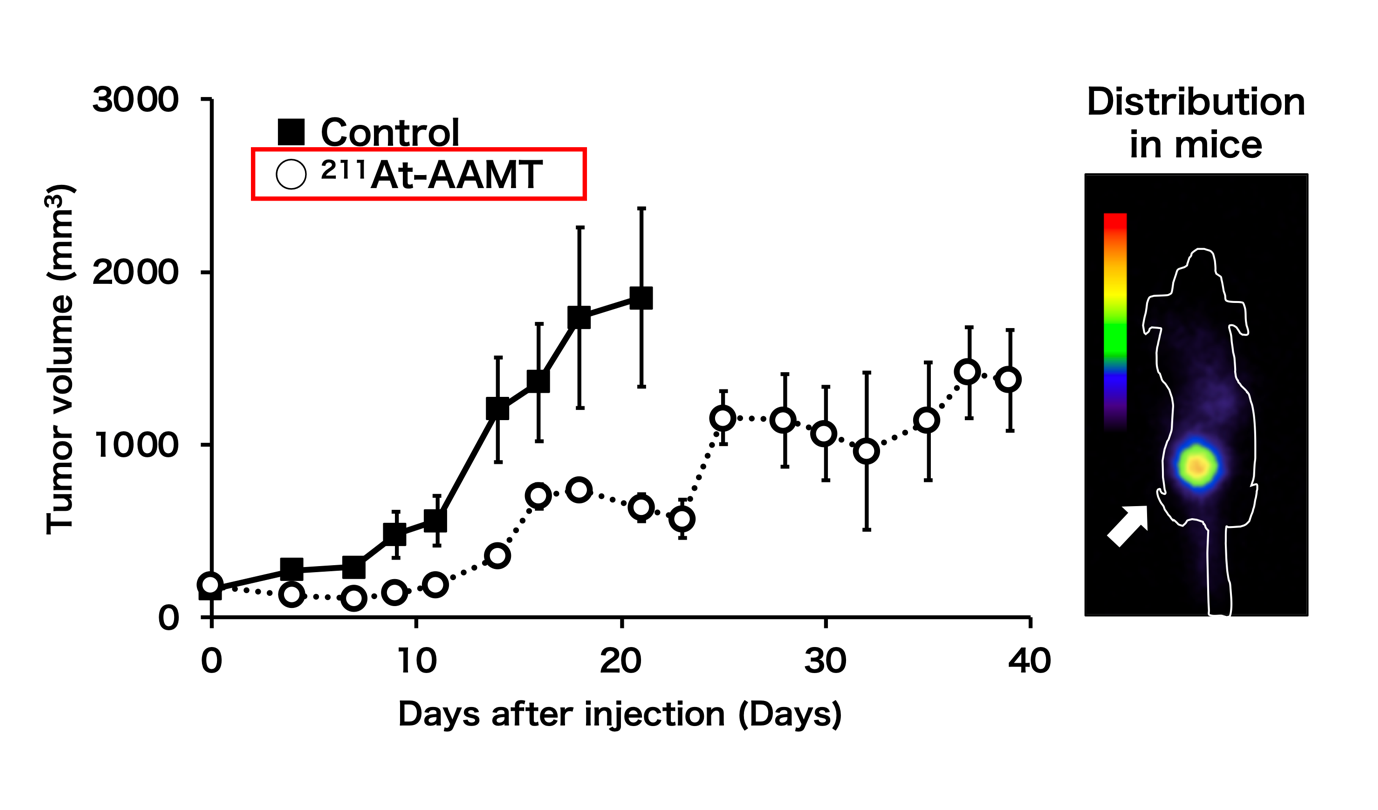
“We found that 211At-labeled α-methyl-L-tyrosine (211At-AAMT) had high affinity for LAT1, inhibited tumor cells, and caused DNA double-strand breaks in vitro,” reports Associate Professor Kazuko Kaneda-Nakashima, lead author. “Extending our research, we assessed the accumulation of 211At-AAMT and the role of LAT1 in an experimental mouse model. Further investigations on a human pancreatic cancer cell line showed that 211At-AAMT selectively accumulated in tumors and suppressed growth. At a higher dose, it even inhibited metastasis in the lung of a metastatic melanoma mouse model.”
Professor Atsushi Shinohara, senior author, explains, “We could establish the efficacy of 211Astatine in the treatment of cancer including advanced and metastatic malignancies, as well as the utility of the amino acid transporter LAT1 as a vehicle for radionuclide therapy. As the drug is delivered cancer-specifically it can attack from inside the cell after being taken in as a nutrient.”
Adding to efficacy is dosing convenience. As an injectable short-range radiopharmaceutical, 211At-AAMT may be administered in outpatient clinics, a huge advantage over conventional radiation protocols, and may even be an alternative to surgery in specific cancers. This approach has immense potential to revolutionize radionuclide therapy of not only pancreatic cancer but other malignancies that lack effective treatment including advanced or metastatic disease.
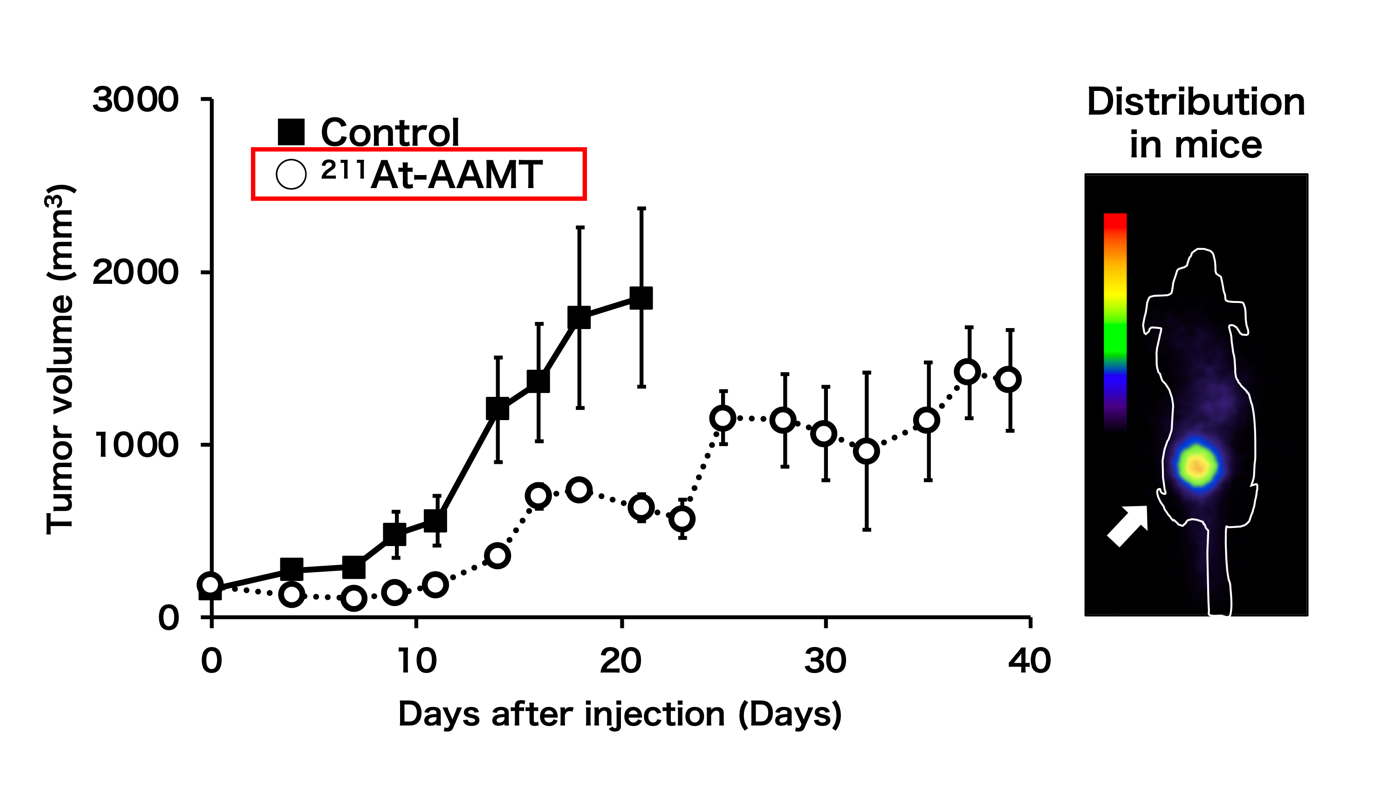
Fig.1: The efficacy of 211At-AAMT using the PANC-1 xenograft model. Tumor growth inhibition by 211At-AAMT(Left). Coronal images of 211At-AAMT in tumor-bearing model(right).
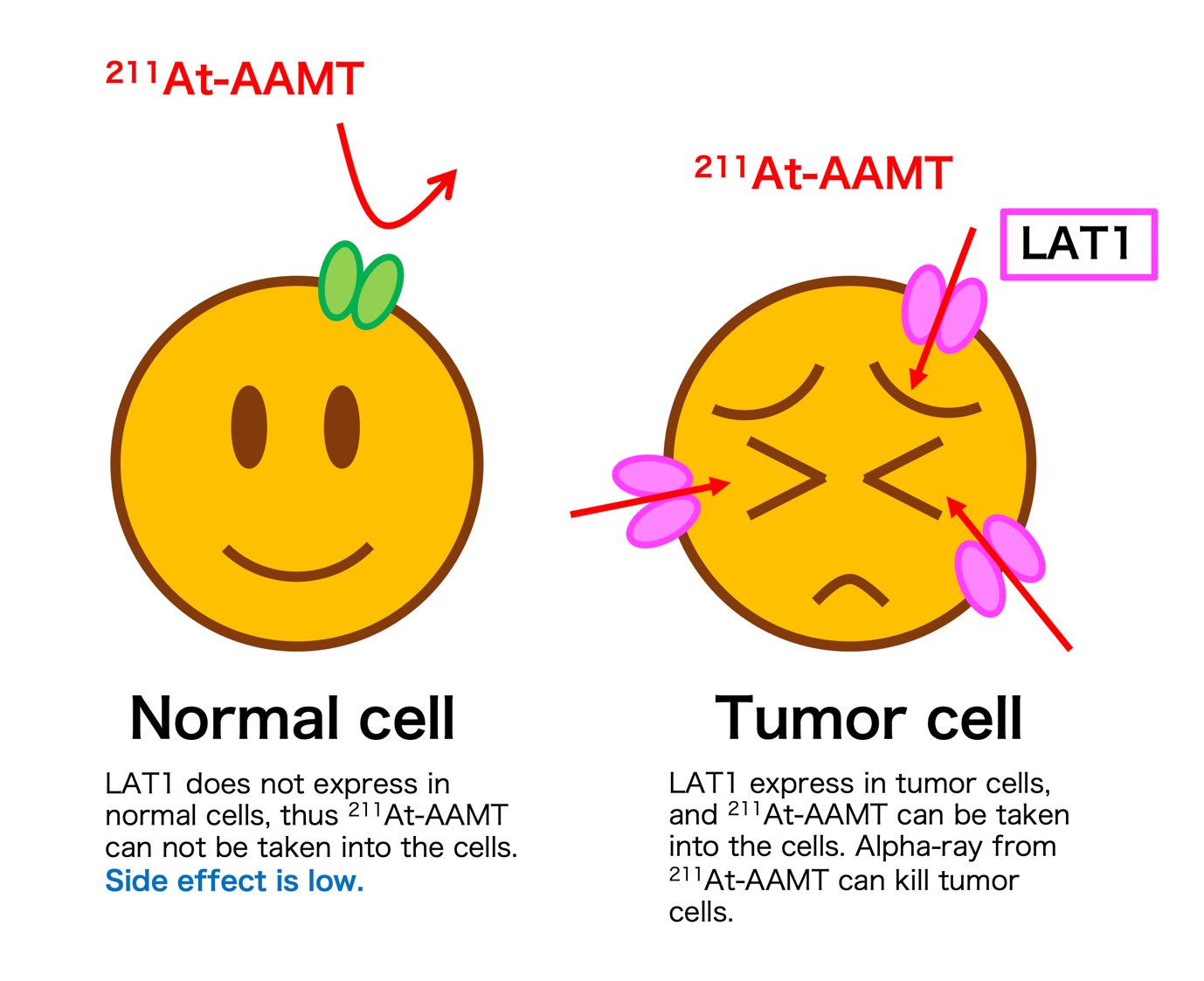
Fig.2: Mechanisms of 211At-AAMT on cancer cells via LAT1.
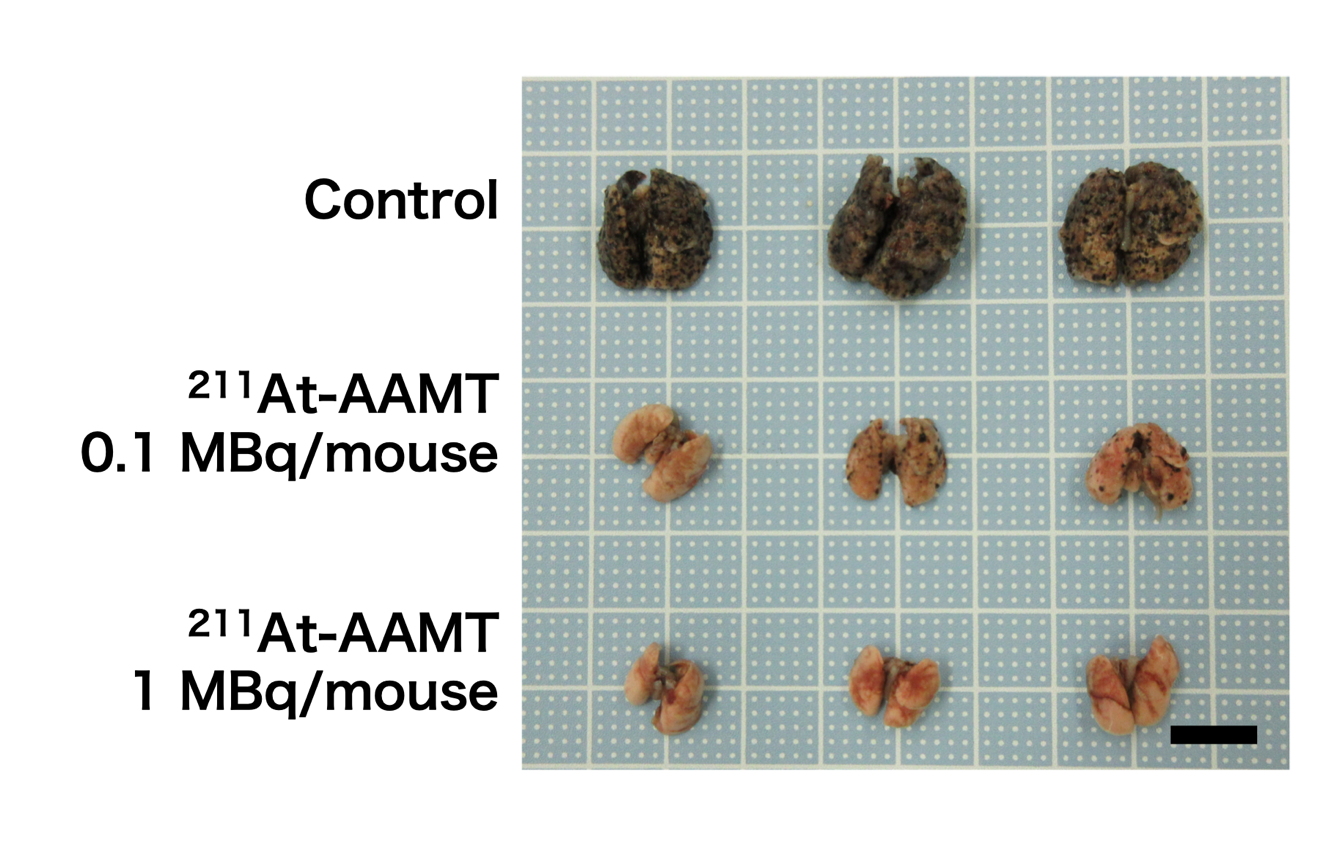
Fig.3: Tumor metastasis inhibition via 211At-AAMT using a B16F10 model. Photos of experimental mice lung.
The article, “α-Emitting cancer therapy using 211At-AAMT targeting LAT1,” was published in Cancer Science at DOI: https://doi.org/10.1111/cas.14761.
Related Links
Institute for Radiation Sciences, Osaka University (link in Japanese)
EurekAlert!
AlphaGalileo
