Rational design of acids based on energy landscape by controlling anion vacancy order
A group of researchers from Kyoto University, Tokyo Institute of Technology, The University of Tokyo, Tsukuba University, Osaka University, the Institute of Materials Structure Science (IMSS) of the Inter-University Research Institute Corporation High Energy Accelerator Research Organization (KEK), the National Institute for Materials Science (NIMS), the Institute for Molecular Science, University of Michigan, and the National Institute of Standards and Technology (NIST), has discovered that the direction or periodicity of anion-vacancy order in strontium vanadium oxide (SrVO3) films can be converted through low-temperature topochemical reactions and stress caused by a mismatch between the underlying substrate and the deposited film.
(Atomic vacancy is an empty space created by the migration of the atom. Oxygen vacancy refers to the vacancy formed by the separation of oxygen atoms (oxygen ions) in the lattice in compounds containing oxygen.)
Since the crystal structure of a material determines its functional properties, the discovery of new structures leads to the acquisition of new functions. Oxynitrides (oxide-nitrides) exhibit attractive properties, but the highly reducing atmosphere of a high-temperature (over 1,000 ℃) reaction with ammonia (ammonolysis) often makes it difficult to obtain the desired structures in inorganic chemistry. Thus, natural superlattice structures are synthesized through solid-state reactions via a so-called powder mixing + sintering process (i.e. a top-down technique).
Thus, in this study, the researchers focused on low-temperature topochemical reactions and stress. Topochemical reactions allow for the synthesis of solid materials with specific structures and properties. Low-temperature (room temperature ~ <500 °C) topochemical chemical reactions are milder approach to various inorganic host materials, allowing access to compounds that cannot be prepared by high-temperature (>1,000℃) methods.
Using SrVO3, an oxide with perovskite structure, the researchers deposited single-crystal (epitaxial) SrVO3 thin films on various single crystalline substrates. SrVO3 thin films grow on a LSAT (Lanthanum Strontium Aluminum Tantalum oxide) substrate in accordance with the size of substrate (lattice constant) because substrate-induced stress exists in SrVO3 thin films.
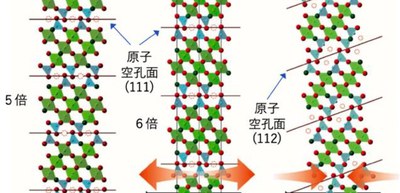
The researchers attempted to topochemically convert SrVO3 films and almost stress-free powder samples to anion-deficient oxynitrides by ammonia treatment. As a result, when SrVO3 powder samples were used, the topochemical O/N exchange with regular (111)p anion-vacancy planes was obtained. When LaAlO3 was used, lattice mismatch with substrate materials yielded a compressive strain and (111)p to (112)p switching arose in the direction of oxygen vacancy. When SrTiO3 was used, the direction of oxygen vacancy remained 111, but the periodicity was elongated five- to six-fold. These results mean that a crystal structure was controlled by stress.
In addition, the researchers showed a low-temperature reaction of SrVO3 topochemically transforming to SrVO2.2N0.6 with regular (111)p anion-vacancy planes. The crystal and electronic structure of SrVO2.2N0.6 was mainly two-dimensional, with conducting octahedral layers separated by insulating tetrahedral layers.
This means that various 2-dimensional metal states can be available by controlling the direction and periodicity of the oxygen-vacancy plane and that the lattice strain can be used to induce and manipulate the anion-vacancy planes and provide a controllable parameter for the development of exotic structural and electronic states in perovskite films.
Strain energy, the energy stored in a material due to deformation, or strain, reaches even to several thousands of degrees Celsius, so unstable structures can be stabilized by external forces or stresses.
In this study, the desired structures were obtained by controlling the energy landscape of matter by stress. It will become possible to obtain desired structures by controlling energy landscape of matter, namely, to obtain more materials that satisfy the desired properties.
Figure 1
Figure 2
The article, “Strain-induced creation and switching of anion vacancy layers in perovskite oxynitrides,” was published in Nature Communications at DOI: https://www.nature.com/articles/s41467-020-19217-7.
Related links
Theory of Electrons in Solids Group
Ochi Masayuki
