Thermostable designed proteins that have a robust folding ability despite the loosened packing
A group of researchers from the Research Center of Integrative Molecular Systems (CIMoS) of the Institute for Molecular Science (IMS), the RIKEN SPring-8 Center (RSC), and the Institute for Protein Research of Osaka University has discovered that de novo designed proteins have the folding ability and high thermal stability even in the absence of tight hydrophobic core packing.
This group had generated various proteins by focusing on optimizing folding: they created the backbone structures by using a set of rules that relate local backbone structures (the length of secondary structure elements and the shape of the loops that connect a β-strand to an α-helix) to preferred tertiary motifs and designed that protein cores were tightly packed with hydrophobic amino acid residues. These proteins, unlike natural proteins, didn’t denature even at 100°C, but it was unknown why they showed very high thermal stability.
Since the de novo protein design rules of this group suggested the importance of local backbone structures than hydrophobic packing in protein cores for stability of de novo designs, the researchers investigated the robustness of folding against the reduction of packing of de novo designed proteins with the highest thermal stability (thermal denaturation: ~130°C) through extensive mutation of large hydrophobic residues (Leucine (Leu), isoleucine (Ile)) to smaller ones Valine (Val) in designed proteins. In order to investigate thermal denaturation, they conducted circular dichroism (CD) measurements of these proteins with reduced and loosened core packing. They measured thermal stability and found that "hub-residues," the residues having contacts with both distant (not adjacent to the secondary structure element containing the mutated residue) and close (adjacent) secondary structures, made a large contribution to the stability.
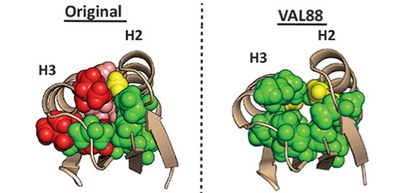
This group constructed a mutant with total 10 substitutions of Leu or Ile with Val, resulting in 30 of the 34 hydrophobic core residues being valine (88% valine in the core, thus referred to as VAL88) and investigated its properties. VAL88 remained folded above 100 °C even with the stability despite the loose core packing.
Using nuclear magnetic resonance (NMR) spectroscopy, the researchers found that VAL88 could fold into the same structure as the original protein. Even after 10 Leu and Ile residues were mutated to Val, this mutant with the core mostly filled with Val folded into the same backbone structure as the original design, with high stability. These results indicate that it was not the hydrophobic core packing, but the backbone structure that contributes to high thermostability.
Increasing thermal stability of proteins is a challenge in using natural proteins for biomaterials and industrial use of enzyme proteins. The mechanisms of thermal stabilization of designed proteins clarified in this study will be useful as a way to increase thermal stability of proteins.
Designing thermostable protein structures is critical in generating proteins with specific functional properties for controlling and designing biological activities and contributing to medical care. Knowledge gained from this study will greatly contribute to creating proteins with new functions.
Figure 1
Figure 2
The article, “Robust folding of a de novo designed ideal protein even with most of the core mutated to valine,” was published in Proceedings of the National Academy of Sciences of the United States of America at DOI: https://doi.org/10.1073/pnas.2002120117.
