How rhodopsin forms highly ordered rows of dimers responsible for single-photon detection clarified
A group of researchers from Hiroshima University, Kobe University, and Osaka University has clarified the mechanisms behind the aligned oligomer formation of rhodopsin (Rh) dimers. Rh is a G protein*–coupled receptor (GPCR) that initiates the phototransduction cascade in retinal disc membrane and a row structure of Rh is essential for efficient and stable signal amplification in the cascade.
*G protein: guanine nucleotide-binding protein
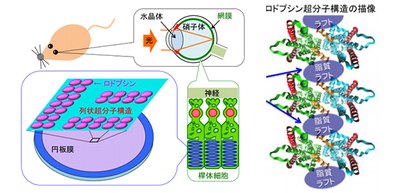
In humans and other vertebrates, light entering the eye is focused by the lens onto the retina. Light reaches to photoreceptor cells (rod cells and cones) and is absorbed by a light-sensitive receptor protein Rh. The outer segments of vertebrate rod photoreceptor cells consist of an ordered stack of membrane disks.
Rh involved in visual phototransduction changes the structure of photoreceptor cells, activates G proteins in the cells, and converts light into an electrical signal. Rh drives intracellular signal transduction systems and amplifies light information.
Rh molecules form rows of dimers in the disk membranes. Disk membranes contain a large amount of unsaturated lipids, cholesterol (accounting for only 8% of total phospholipid), and saturated lipids.
Previously, researchers from Kobe University reported the presence of rafts in disk membranes and indicated that rafts had an important role in light signal conversion and that Rh conferred a high lipid raft affinity (raftophilicity).
They also found that Rh self-organized 100 nm-order short-lived clusters in disk membranes, suggesting that Rh-dimer raftophilicity contributes to the organization of the higher order structure.
By comparing two types of Rh model structures, the researchers found that disk membranes were segregated into two concentric regions, i.e. a raftophilic center with confined rhodopsin-clusters, and a non-raftophilic (raftophobic) periphery. They believed that the shapes of Rh-oligomers differed among the models.
In this study, based on the findings from experiments by Kobe University and Osaka University, researchers from Hiroshima University performed coarse-grained molecular dynamics simulations of disk membrane models containing unsaturated lipids, saturated lipids with cholesterol, and Rh-dimers.
These simulations revealed that only the dimers that have raftophilic regions at the center could form ordered row structures of Rh-dimers and that the nano-sized lipid raft domains acted as a “glue” to organize the long row structures of Rh-dimers.
These findings provide an insight into how the row structures of Rh-dimers work when Rh relays send signals to other proteins, leading to the understanding of how light is converted into an electrical signal and is then transmitted to the brain. They also show how the oligomerization-induced raftophilicity of Rh promotes spontaneous formation of raftophilic Rh-clusters, providing a clue to understanding mechanisms of self-organization and localization of proteins and lipids.
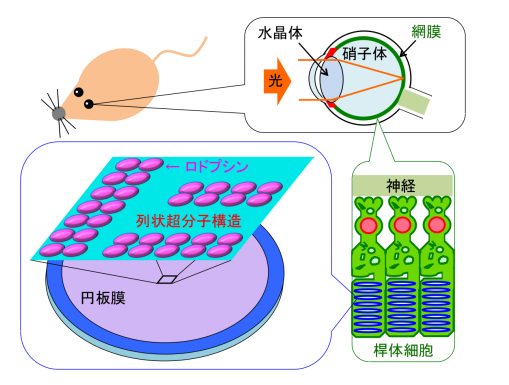
Figure 1
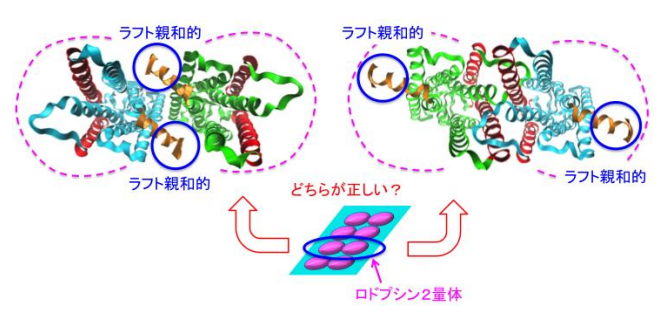
Figure 2
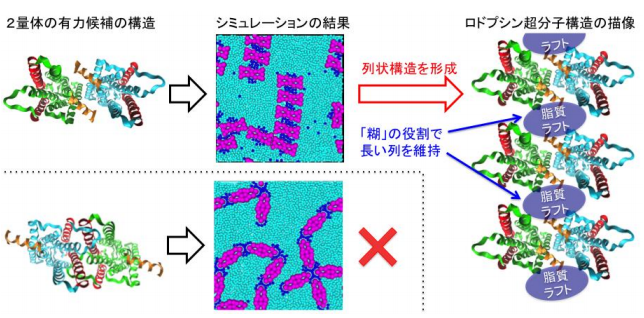
Figure 3
The article, " Affinity of rhodopsin to raft enables the aligned oligomer formation from dimers: Coarse-grained molecular dynamics simulation of disk membranes," was published in PLOS ONE at DOI: https://doi.org/10.1371/journal.pone.0226123.
Related links
