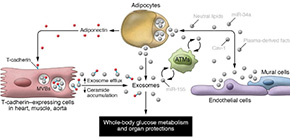
Metabolic regulation by adipose tissue via adiponectin and exosomes
A group of researchers from Osaka University clarified that adiponectin (APN) stimulated muscle regeneration through T-cadherin (T-cad) to promote exosome production and played an important role in regulating exosome secretion and whole-body metabolism.
APN is a secreting factor from adipocytes and its circulating level decreases in metabolic abnormalities, such as obesity, diabetes, and hypertension. T-cad, an adiponectin-binding protein, is abundantly expressed in skeletal muscles, cardiac muscles, and vascular endothelial cells.
It was believed that hormones and cytokines directly served to convey information to organs and regulated whole-body metabolism and that adipose tissue played a central role in regulating whole-body energy by storing energy and secreting adipocytokines. However, it was recently reported that exosome production by adipose tissue contributed to the regulation of whole-body metabolism.
Skeletal muscle has remarkable regenerative potential and its decline with aging is thought to be one of the causes of loss of muscle mass and compromised quality of life in the elderly. Metabolic abnormalities such as obesity are linked with decline of muscle regeneration and plasma levels of APN are low in such metabolic conditions. However, plasma levels of APN inversely correlate with muscle weakness in elderly people, muscle mass and strength especially in heart failure patients.
This group has examined whether APN impacts muscle regeneration after cardiotoxin-induced muscle injury in mice. Preserving muscle regeneration ability may slow the development of the loss of muscle mass in the elderly.
The group found that APN from adipose tissue stimulates T-cad cells, regulating exosome production. This suggests that regulating exosome production directly by adipose tissue or via APN plays an important role in endocrine secretion and metabolism regulation.
Moving forward, studying the level of exosomes in the blood will lead to the development of diagnosis and treatment of metabolic diseases and their complications.

Figure 1
The article, "Interorgan communication by exosomes, adipose tissue, and adiponectin inmetabolic syndrome," was published in The Journal of Clinical Investigation at DOI: https://doi.org/10.1172/JCI129193 .
