Crystal structure of Wnt proteins involved in the development of various diseases unveiled
Will accelerate research on mammalian organogenesis, regenerative medicine, and cancer
A team of researchers led by Prof. Junichi TAKAGI from the Institute for Protein Research of Osaka University crystalized a Wnt–Fz complex, clarifying its crystal structure. Wnt is a protein essential for the development of multicellular creatures, including humans, and Frizzled (Fz) is a receptor protein that serves as a receptor in the Wnt signaling pathway.
Three-dimensional (3D) cell culture (also known as “culture of organoids”) that can more faithfully reproduce in vivo function has gained a lot of attention as a means to understand cancer mechanisms and develop personalized therapies for diverse carcinoma.
Factors necessary for maintaining and proliferating stem cells (including organoids) are Wnt proteins, secreted proteins that regulate cell fate specification. Misregulation of Wnt signaling pathways that activate stem cells can result in various diseases, including osteoporosis and cancer. Thus, it is desirable to clarify a molecular structure at the signal initiation stage after Wnt proteins bind to receptors on cells.
The clarification will accelerate the development of medicines for regulating the Wnt signaling pathway; however, Wnt proteins are not water-soluble due to their strong hydrophobic property by a covalent lipid modification. For this reason, it was very difficult to produce Wnt proteins through genetic recombination and no information about crystal structure of a mammalian Wnt protein necessary for studying human stem cells was available.
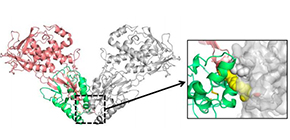
The team extensively optimized the Wnt and Fz constructs, conducting various chemical modifications to ensure high expression yields, enhanced solubility, and sample homogeneity. As a result, they obtained crystals of lysine-methylated and deglycosylated human Wnt3 (hWnt3)–mFz8 Cys-rich domain (CRD) complex.
By intentionally removing a hydrophobic segment in Wnt3, they also obtained and crystallized water-soluble and stable region in the Wnt3-Fz8 complex. Performing diffraction experiments at beamline BL44XU of SPring-8, they succeeded in determining the complex’s structure at an atomic resolution level of 2.9Å.
This team demonstrated that Fz dimerization by Wnt initiated signaling and recruited more low-density lipoprotein receptor (LDLR)-related 6 (LRP6) into a ternary complex, which is a great advancement in the clarification of Wnt signaling pathways. This will accelerate the comprehensive understanding of Wnt signal mechanisms and the development of treatments for various diseases using the mechanisms.
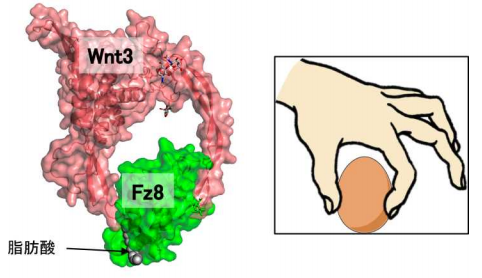
Figure 1

Figure 2
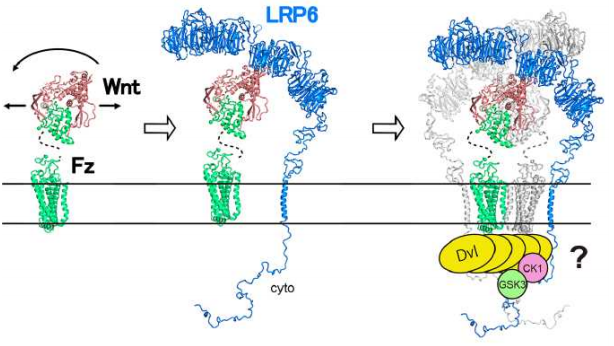
Figure 3
The article, “Crystal structure of mammalian Wnt–frizzled complex” was published in Nature Structural & Molecular Biology at DOI: https://doi.org/10.1016/j.celrep.2019.03.106 .
Related links
