New Strategy for Multiple Myeloma Immunotherapy
Osaka University study unveils new target for monoclonal antibody-based treatment of cancer
In recent decades monoclonal antibody-based treatment of cancer has been established as one of the most successful therapeutic strategies for both solid tumors and blood cancers. Monoclonal antibodies (mAb), as the name implies, are antibodies that are made by clonal cells derived from a single parent cells and therefore share the identical amino acid sequences.
One of the leading technologies to emerge in mAb-based treatment is CAR-T, where CAR stands for “chimeric antigen receptor”, and T represents T cells, a type of white blood cells that have pivotal roles in immune defenses. CARs are produced by combining together the gene for an antibody that recognizes a tumor antigen with the gene for a receptor that resides on the surface of the T cells; insert this new gene into a T cell and it will be precisely targeted at the tumor.
Theoretically, new antigens – molecules capable of inducing an immune response to produce an antibody – that arise from cancer-specific mutations of cell-surface proteins are excellent targets. However, mAb therapy targeting such antigens is impractical because of these proteins’ vast diversity within and between individual tumors, which renders identifying new cancer-specific target antigens difficult.
However, such challenges have driven researchers centered at Japan’s Osaka University to think outside of the box; cancer-specific antigen formed by the modification of proteins during or after synthesis, such as glycosylation (attachment of sugar moieties to protein) or conformational changes, might have been missed in previous analyses. The team believed new antigen epitopes, which is the part of an antigen recognized by the immune cells, could be discovered by thoroughly searching for cancer-specific mAbs and characterizing the antigens they recognize.
“We applied this strategy to identify novel therapeutic targets for multiple myeloma (MM), a cancer that forms in a type of white blood cell called a plasma cell,” explains Naoki Hosen, lead author of the study, which was recently published in Nature Medicine. “Despite advances in MM treatment, relapse remains common. As such, there is an ongoing need for new therapeutic approaches, including mAb-based therapies.”
The team screened more than 10,000 anti-MM mAb clones and identified MMG49 as an MM-specific mAb specifically recognizing a subset of integrin β7, a cell-surface receptors that facilitate cell-extracellular matrix adhesion. MMG49 reacted to MM cells, but not other bone marrow cell types in MM patient samples. This prompted the researchers to design a CAR that incorporates a fragment derived from MMG49. The resulting MMG49 CAR T was found to have anti-MM effects without damaging normal blood cells.
“Our results also demonstrate that the active conformer of integrin β7 can serve as an immunotherapeutic target against MM, even though the expression of the protein itself is not specific to MM,” study coauthor Yukiko Matsunaga says. “Therefore it’s highly plausible that there are other cancer immunotherapeutic targets that have yet to be identified in many cell-surface proteins that undergo conformational changes, even if the expression of the proteins themselves is not cancer-specific.”
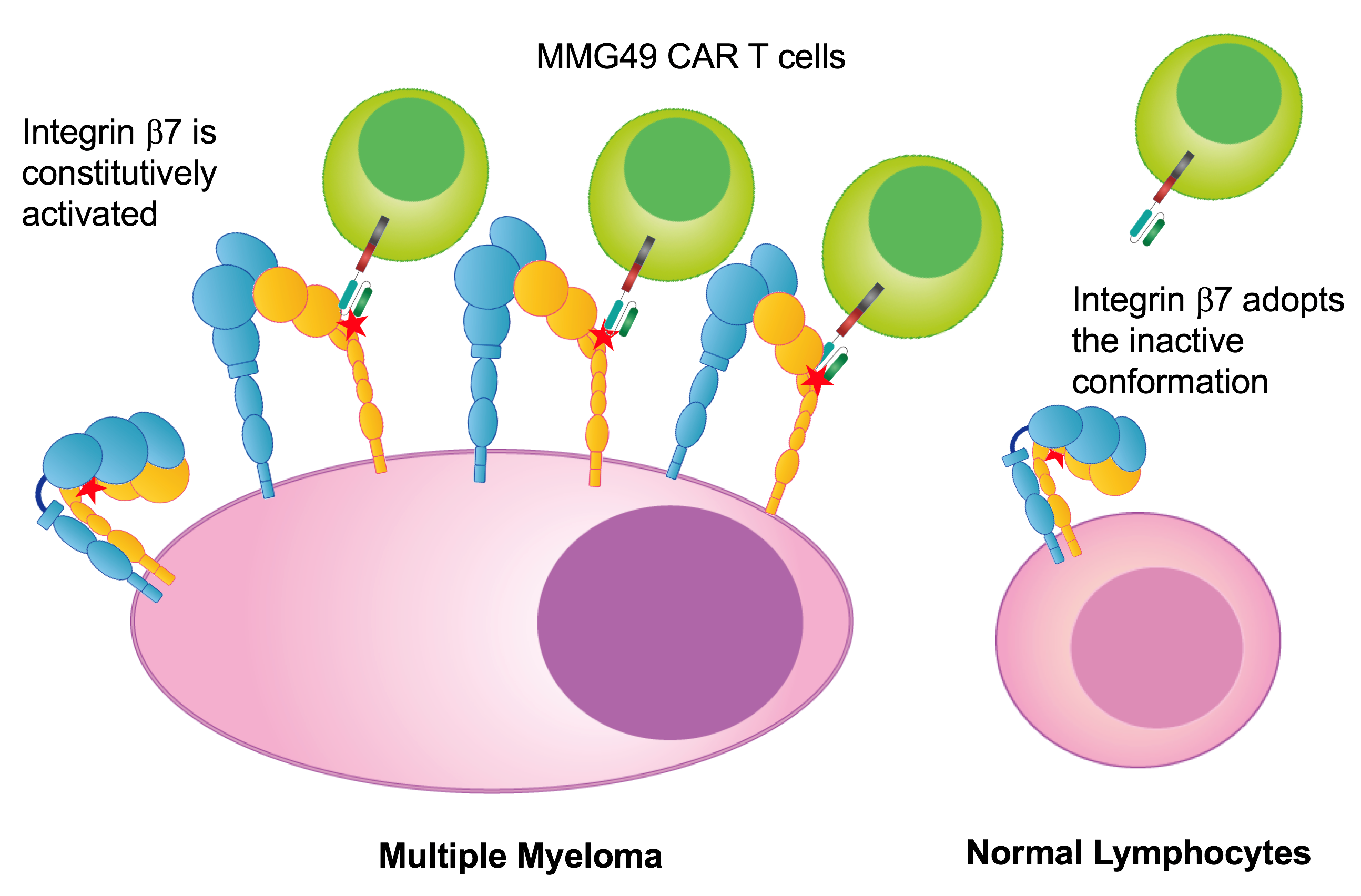
Fig. 1. MMG49 CAR T cells target the activated conformation of integrin b7 expressed on MM cells (credit: Osaka University)
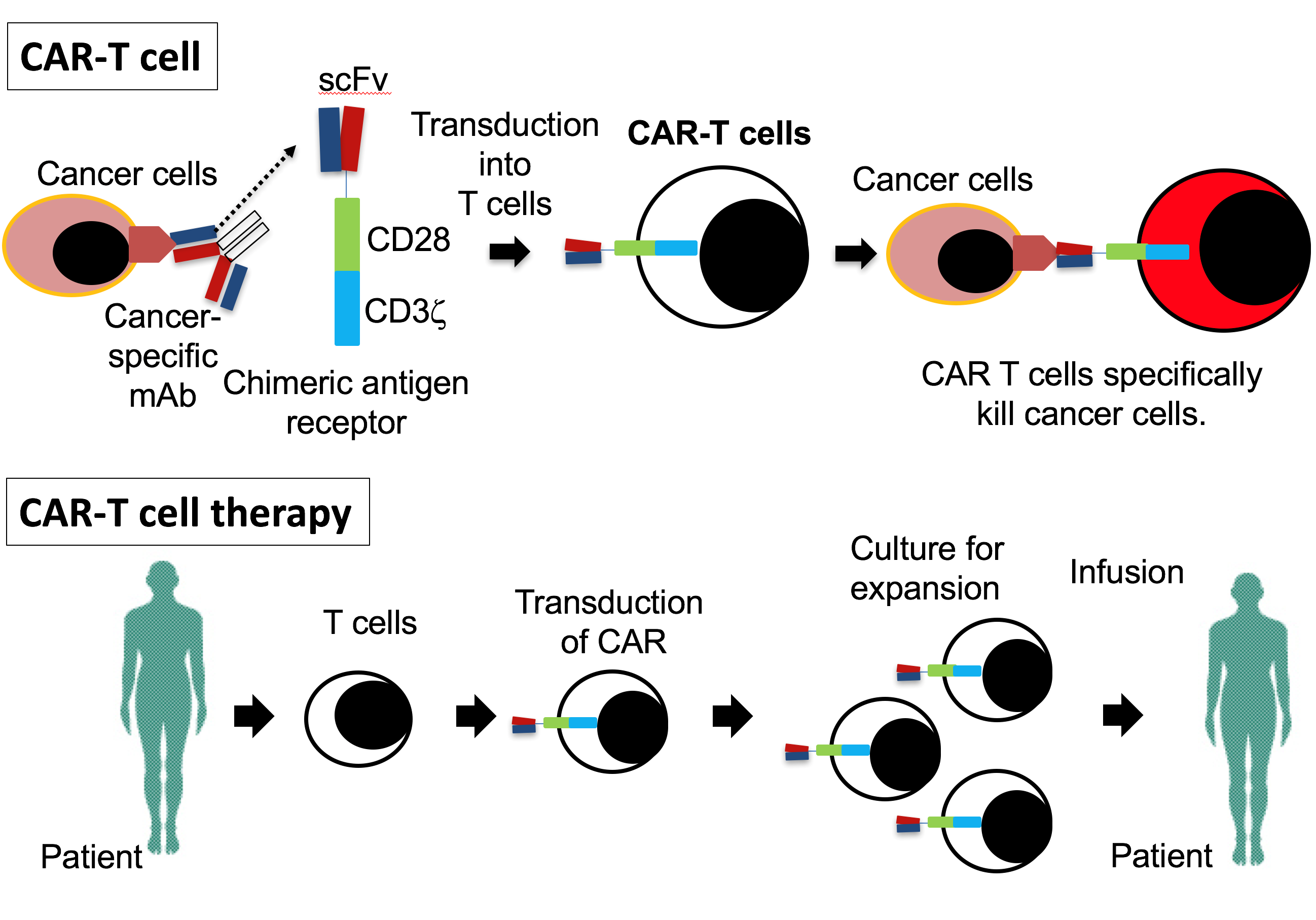
Fig. 2. Outline of CAR T cell therapy (credit: Osaka University)
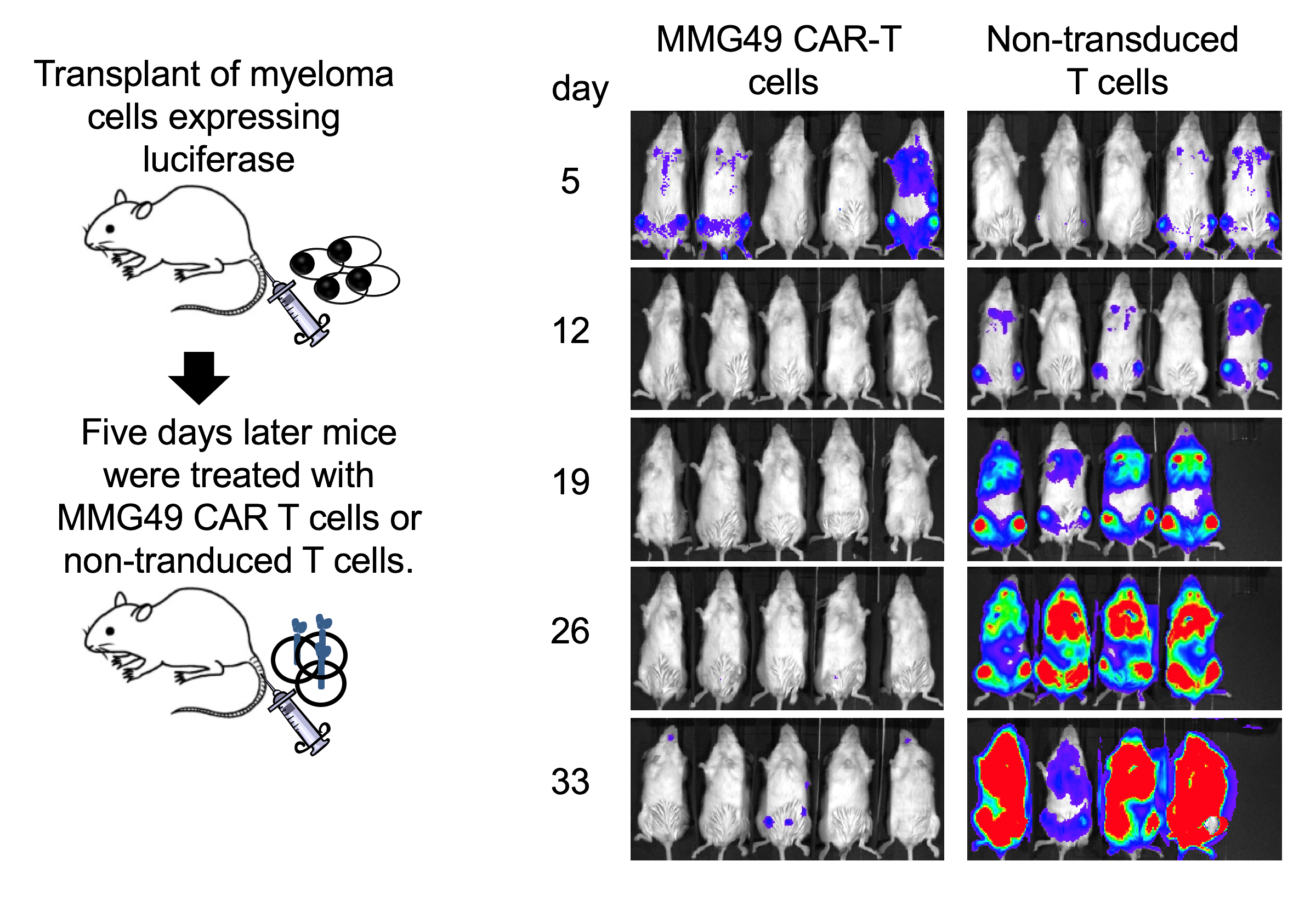
Fig. 3. Anti-myeloma activity of MMG49 CAR T cells (credit: Osaka University)
To learn more about this research, please view the full research report entitled " The activated conformation of integrin β7 is a novel multiple myeloma–specific target for CAR T cell therapy " at this page of Nature Medicine .
Related links
- Laboratory of Immuno-Oncology and Hematology, Division of Health Science, Graduate School of Medicine, Osaka University
- Department of Immunopathology, Research Institute for Microbial Diseases, Osaka University
- Laboratory of Protein Synthesis and Expression, Institute of Protein Research, Osaka University
