Mechanism behind catalytic hydroxylation of stable C-H bonds using artificial enzyme at room temperature elucidated
Will lead to efficient use of natural carbon resources
A group of researchers led by Assistant Professor OOHORA Koji and Professor HAYASHI Takashi at the Graduate School of Engineering, Osaka University, together with Professor SHIRO Yoshitsugu and Chief Researcher SUGIMOTO Hiroshi at the RIKEN Spring-8 Center, clarified that the active species of a hydroxylation reaction (changing C-H bonds to O-H bonds) catalyzed by an artificial enzyme, or myoglobin manganese reconstituted with porphycene, was the Oxomanganese(V) species (Mn V -oxo species), a world first.
The C−H bonds are very strong and unreactive, so it was difficult to activate them to produce functionalized products. Native enzymes to facilitate hydroxylation reactions have drawn attention in recent years; however, the identification of the active species in such enzymes has been thought to be difficult. For this reason, the identification of active species has been limited in some systems, although it was important for understanding mechanism behind reactions of enzyme and improving such reactions.
Using a stopped flow apparatus, this group clarified that the active species of artificial enzymes that contain manganese, which efficiently changes stable CH bonds to C-OH bonds, was the Mn V -oxo species. This group also demonstrated that the active species obtained could hydroxylate stable C-H bonds at room temperature without requiring complex protein environments as native enzymes do. This group’s achievement has enabled the designing of artificial enzymes, which will allow for stereoselective/regioselective hydroxylation of complex organic compounds as well as efficient conversion of abundant natural carbon resources into useful chemicals.
Abstract
A mechanistic study of H 2 O 2 -dependent C–H bond hydroxylation by myoglobin reconstituted with a manganese porphycene was carried out. The X-ray crystal structure of the reconstituted protein obtained at 1.5 Å resolution reveals tight incorporation of the complex into the myoglobin matrix at pH 8.5, the optimized pH value for the highest turnover number of hydroxylation of ethylbenzene. The protein generates a spectroscopically detectable two-electron oxidative intermediate in a reaction with peracid, which has a half-life up to 38 s at 10 °C. Electron paramagnetic resonance spectra of the intermediate with perpendicular and parallel modes are silent, indicating formation of a low-spin Mn V -oxo species. In addition, the Mn V -oxo species is capable of promoting the hydroxylation of sodium 4-ethylbenzenesulfonate under single turnover conditions with an apparent second-order rate constant of 2.0 M –1 s –1 at 25 °C. Furthermore, the higher bond dissociation enthalpy of the substrate decreases the rate constant, in support of the proposal that the H-abstraction is one of the rate-limiting steps. The present engineered myoglobin serves as an artificial metalloenzyme for inert C–H bond activation via a high-valent metal species similar to the species employed by native monooxygenases such as cytochrome P450.
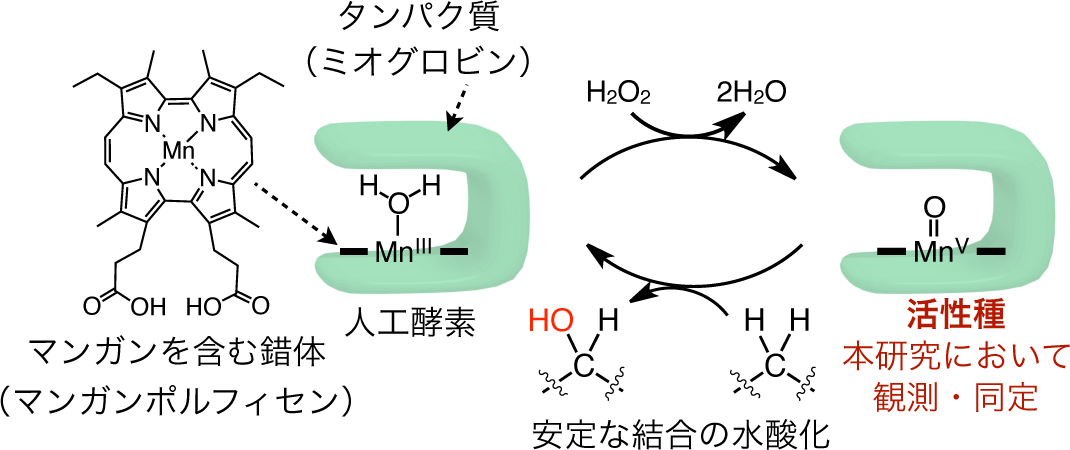
Figure 1
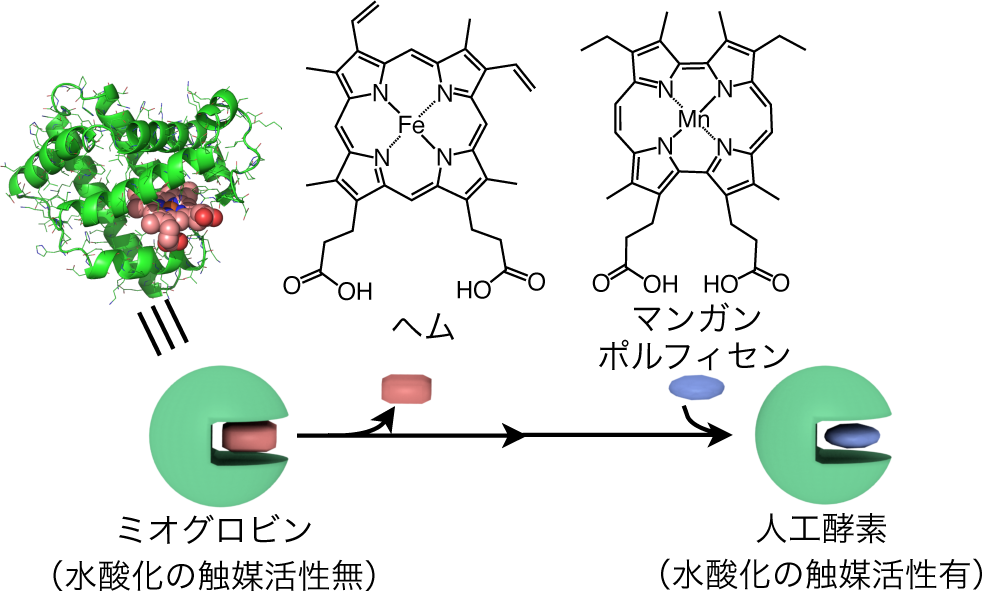
Figure 2
To learn more about this research, please view the full research report entitled " Manganese(V) Porphycene Complex Responsible for Inert C–H Bond Hydroxylation in a Myoglobin Matrix " at this page of Journal of the American Chemical Society .
Related links
