
Solar Material for Producing Clean Hydrogen Fuel
Osaka University researchers create new material based on gold and black phosphorus to produce clean hydrogen fuel using the full spectrum of sunlight
Global climate change and the energy crisis mean that alternatives to fossil fuels are urgently needed. Among the cleanest low-carbon fuels is hydrogen, which can react with oxygen to release energy, emitting nothing more harmful than water (H2O) as the product. However, most hydrogen on earth is already locked into H2O (or other molecules), and cannot be used for power.
Hydrogen can be generated by splitting H2O, but this uses more energy than the produced hydrogen can give back. Water splitting is often driven by solar power, so-called “solar-to-hydrogen” conversion. Materials like titanium oxide, known as semiconductors with the wide band-gap, are traditionally used to convert sunlight to chemical energy for the photocatalytic reaction. However, these materials are inefficient because only the ultraviolet (UV) part of light is absorbed—the rest spectrum of sunlight is wasted.
Now, a team in Osaka University has developed a material to harvest a broader spectrum of sunlight. The three-part composites of this material maximize both absorbing light and its efficiency for water splitting. The core is a traditional semiconductor, lanthanum titanium oxide (LTO). The LTO surface is partly coated with tiny specks of gold, known as nanoparticles. Finally, the gold-covered LTO is mixed with ultrathin sheets of the element black phosphorus (BP), which acts as a light absorber.
“BP is a wonderful material for solar applications, because we can tune the frequency of light just by varying its thickness, from ultrathin to bulk,” the team leader Tetsuro Majima says. “This allows our new material to absorb visible and even near infrared light, which we could never achieve with LTO alone.”
By absorbing this broad sweep of energy, BP is stimulated to release electrons, which are then conducted to the gold nanoparticles coating the LTO. Gold nanoparticles also absorb visible light, causing some of its own electrons to be jolted out. The free electrons in both BP and gold nanoparticles are then transferred into the LTO semiconductor, where they act as an electric current for water splitting.
Hydrogen production using this material is enhanced not only by the broader spectrum of light absorption, but by the more efficient electron conduction, caused by the unique interface between two dimensional materials of BP and LTO. As a result, the material is 60 times more active than pure LTO.
“By efficiently harvesting solar energy to generate clean fuel, this material could help to clean up the environment,” Majima says. “Moreover, we hope our study of the mechanism will spur new advances in photocatalyst technology.”
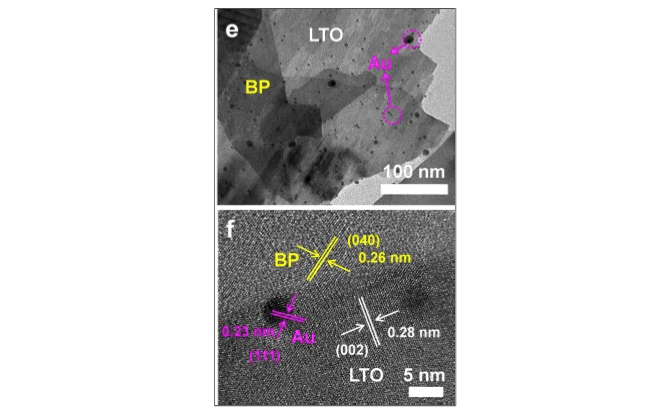
Figure 1. Electron microscope images of visible-NIR light responsible photocatalyst composed with black phosphorous (BP), lanthanum titanate (LA 2 Ti 2 O 7 , LTO), and gold nanoparticles (Au). (© Zhu M, Cai X, Fujitsuka M, Zhang J, Majima T, Angewandte Chemie: International Edition 56 (2017), doi: 10.1002/anie.201612315)
To learn more about this research, please view the full research report entitled " Au/La 2 Ti 2 O 7 Nanostructures Sensitized with Black Phosphorus for Plasmon-Enhanced Photocatalytic Hydrogen Production in Visible and Near-Infrared Light " at this page of Angewandte Chemie: International Edition.
Related link
Majima Lab, Department of Molecular Excitation Chemistry, The Institute of Scientific and Industrial Research, Osaka University
http://www.sanken.osaka-u.ac.jp/labs/mec/english.html
