Photocatalyst makes hydrogen production 10 times more efficient
A group of Japanese researchers has developed a photocatalyst that increases hydrogen production tenfold
Hydrogen is an alternative source of energy that can be produced from renewable sources of sunlight and water. A group of Japanese researchers has developed a photocatalyst that increases hydrogen production tenfold.
The discovery was made by a joint research team led by Associate Professor TACHIKAWA Takashi (Molecular Photoscience Research Center, Kobe University) and Professor MAJIMA Tetsuro (Institute of Scientific and Industrial Research, Osaka University). Their findings were published on April 6 in the online version of Angewandte Chemie International Edition.
When light is applied to photocatalysts, electrons and holes are produced on the surface of the catalyst, and hydrogen is obtained when these electrons reduce the hydrogen ions in water. However, in traditional photocatalysts the holes that are produced at the same time as the electrons mostly recombine on the surface of the catalyst and disappear, making it difficult to increase conversion efficiency.
Professor Tachikawa’s research group developed a photocatalyst made of mesocrystal, deliberately creating a lack of uniformity in size and arrangement of the crystals. This new photocatalyst is able to spatially separate the electrons and electron holes to prevent them recombining. As a result, it has a far more efficient conversion rate for producing hydrogen than conventional nanoparticulate photocatalysts (approximately 7%).
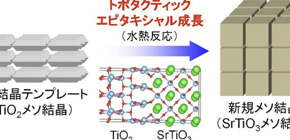
The team developed a new method called “Topotactic Epitaxial Growth” that uses the nanometer-sized spaces in mesocrystals. Based on this synthesis method they were able to synthesize strontium titanate (SrTiO 3 ) from a compound with a different structure, titanium oxide (TiO 2 ), using a simple one-step hydrothermal reaction. By lengthening the reaction time, they could also grow larger particles near the surface while preserving their crystalline structure.
When they attached a co-catalyst to the synthesized mesocrystal and applied ultraviolet light in water, the reaction occurred with approximately 7% light energy conversion efficiency. Under the same conditions, SrTiO 3 nanoparticles which had not been converted into mesocrystals reached a conversion efficiency of less than 1%, proving that the reaction efficiency increased tenfold under the mesocrystal structure. When each particle was examined under a fluorescent microscope, the team found that the electrons produced during the reaction gathered around the larger nanocrystals.
When exposed to ultraviolet light, the electrons in this newly-developed photocatalyst move smoothly between the nanoparticles inside the mesocrystal, gather around the larger nanocrystals generated on the surface of the crystal, and efficiently reduce the hydrogen ions to create hydrogen.
The discovery of this powerful photocatalyst started with the researchers’ idea to “deliberately break down the ordered structure of mesocrystals”, a concept that could be applied to other materials. The strontium titanate used this time is a cubic crystal, which means there is no variation in molecular adsorption or the reaction strength for each crystal plane. By regulating the size and spatial arrangement of the nanocrystals, which form the building blocks for this structure, it may be possible to greatly increase the light energy conversion efficiency of the existing system.
Using these findings, the research group plans to apply mesocrystal technology to realizing the super-efficient production of hydrogen from solar energy. The perovskite metal oxides, including strontium titanate, the target of this study, are the fundamental materials of electronic elements, so their results could be applied to a wide range of fields.
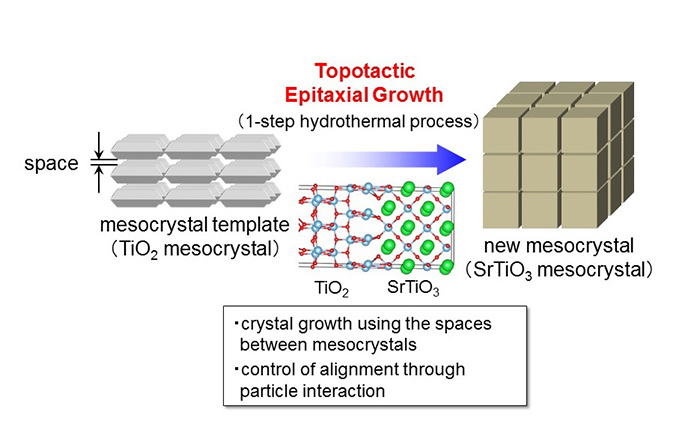
Figure 1. Synthesis of SrTiOmeso crystals by Topotactic Epitaxial Growth
Using the spaces in mesocrystals, SrTiO 3 mesocrystals are synthesized from TiO 2 mesocrystals using a one-step hydrothermal process.

Figure 2: The structure of SrTiO 3 mesocrystals
(a) Electron microscopy image of SrTiO 3 mesocrystals obtained during a 24-hour hydrothermal reaction. The nanocrystals are arranged facing the same direction.
Electron microscopy image of SrTiO 3 mesocrystals after 48-hours of hydrothermal reaction. The surface nanocrystals have grown in size. We can see that regardless of size and position, the nanocrystals forming the mesocrystal face the same direction.
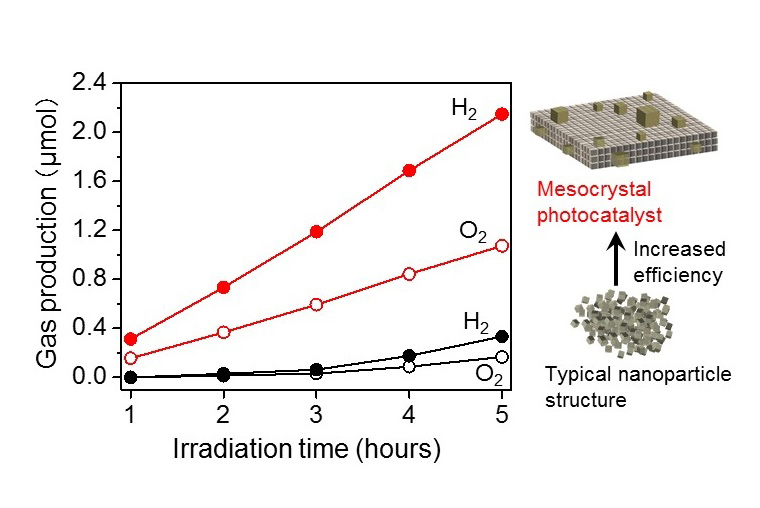
Figure 3: Water-splitting reaction under ultraviolet light
Compared to the SrTiO 3 nanocrystals (black) which are mostly the same structure and size, the SrTiO 3 mesocrystals (red) demonstrate a high water-splitting efficiency. Mixed-oxide of rhodium and chromium was used as the co-catalyst.
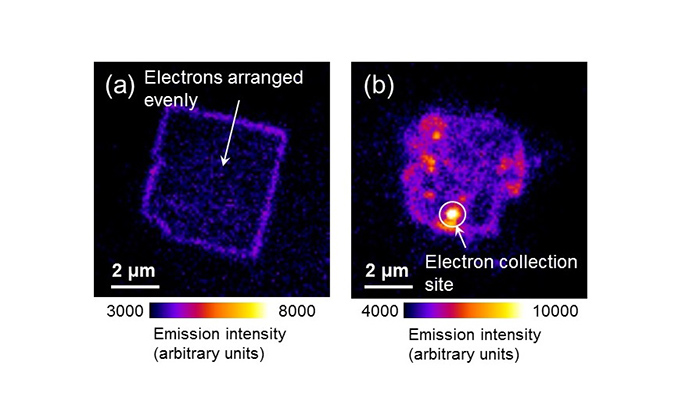
Figure 4: SrTiO 3 mesocrystal light emission
(a) Light emission from SrTiO 3 mesocrystals obtained in a 24-hour hydrothermal reaction. A weak light is seen equally throughout apart from the crystal edges.
(b) Light emission from SrTiO 3 mesocrystals obtained in a 48-hour hydrothermal reaction. They shine strongly due to the electrons gathered around the large crystals on the surface. The light emitted has a wavelength of 405nm.

Figure 5: Hydrogen synthesis in SrTiO 3 mesocrystals
(a) The electrons produced under ultraviolet light move between the nanoparticles in the mesocrystal, gather around the comparatively large nanocrystals exposed on the surface, and reduce the hydrogen ions.
(b) In the nanocrystals that aggregated with a disordered structure, most of the electrons and electron holes recombine and disappear, so the amount of hydrogen produced is low.
To learn more about this research, please view the full research report entitled “ Topotactic Epitaxy of SrTiO 3 Mesocrystal Superstructures with AnisotropicConstruction for Efficient Overall Water Splitting ” at this page of the Angewandte Chemie International Edition website.
Related links
