Identification of a molecule involved in transmucosal antigen uptake
Will lead to the development of needle-free mucosal vaccines
A group of researchers led by Professor KIYONO Hiroshi at the Institute of Medical Science, The University of Tokyo, and Specially Appointed Associate Professor SATO Shintaro at the Research Institute for Microbial Diseases, Osaka University, identified Allograft inflammatory factor1 (Aif1) as a molecule that contributed to the antigen-uptake function of microfold cells (M cells).
Since M cells, membranous epithelial cells, have shorter and thinner microvilli than those of epithelial cells in surrounding areas, they easily take up foreign antigens from the intestinal luminal side. In addition, M cells express receptors to various microorganisms on the luminal side and contribute to the efficient uptake of these particles. However, the molecules that promote transcytosis (antigen transport) by M cells was not fully identified.
This group’s achievements suggest that Aif1 induces membrane ruffling on the intestinal luminal side at the time of uptake of foreign antigens through remodeling of actin. (Actin is responsible for cell movement.) If expression and function of Aif1 become controlled, it will likely increase the efficiency of antigen uptake toward the development of mucosal vaccines that do not need syringes, and prevent infection by inhibiting the transmission of pathogenic microbes. It is also anticipated that this will lead to the establishment of mucosal vaccine delivery methods.
Abstract
M cells in follicle-associated epithelium (FAE) are specialized antigen-sampling cells that take up intestinal luminal antigens. Transcription factor Spi-B regulates M-cell maturation, but the molecules that promote transcytosis within M cells are not fully identified. Here we show that mouse allograft inflammatory factor 1 (Aif1) is expressed by M cells and contributes to M-cell transcytosis. FAE in Aif1 −/− mice has suppressed uptake of particles and commensal bacteria, compared with wild-type mice. Translocation of Yersinia enterocolitica, but not of Salmonella enterica serovar Typhimurium, leading to the generation of antigen-specific IgA antibodies, is also diminished in Aif1-deficient mice. Although β1 integrin, which acts as a receptor for Y. enterocolitica via invasin protein, is expressed on the apical surface membranes of M cells, its active form is rarely found in Aif1 −/− mice. These findings show that Aif1 is important for bacterial and particle transcytosis in M cells.

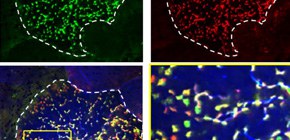
Figure 1.
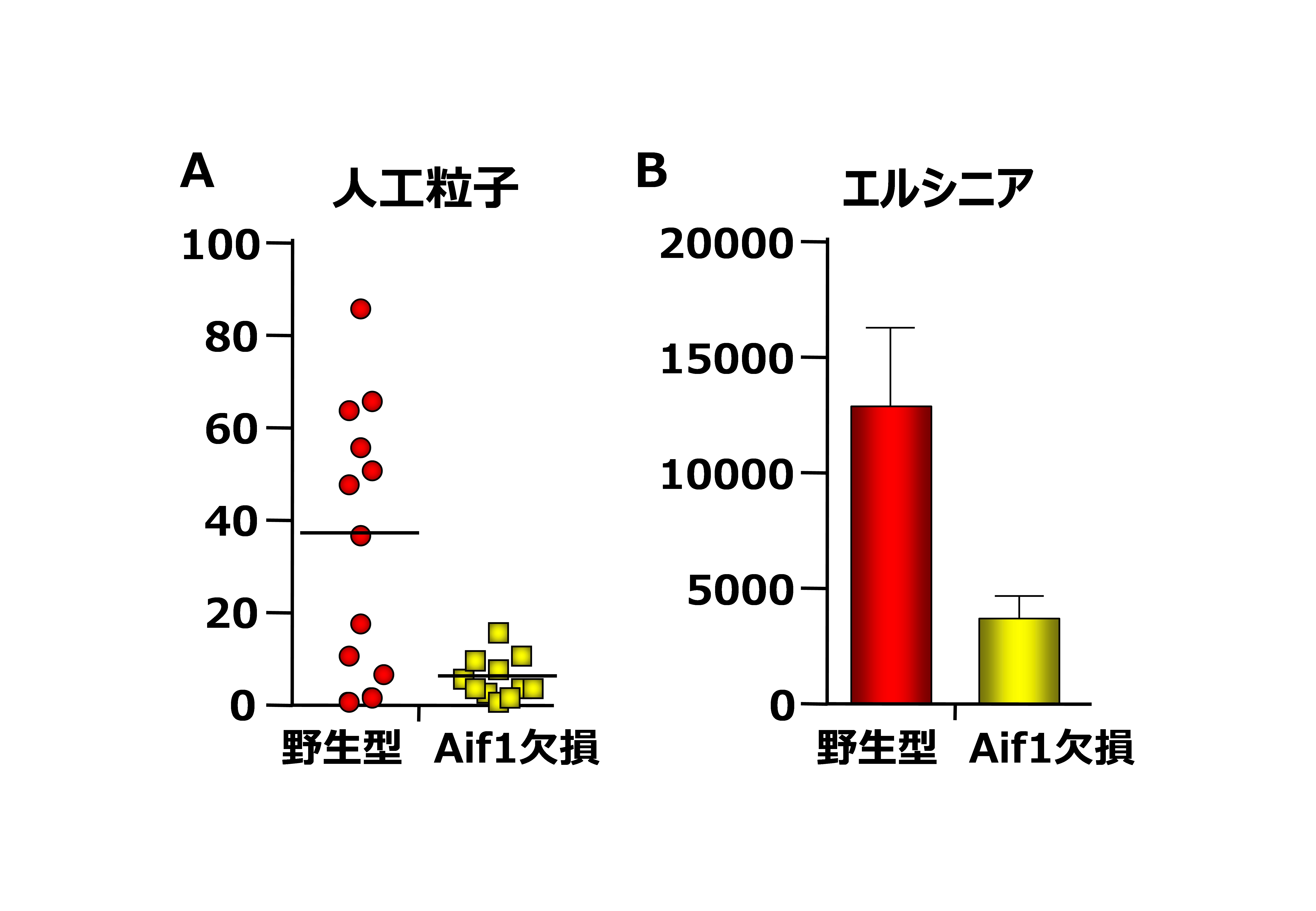
Figure 2.
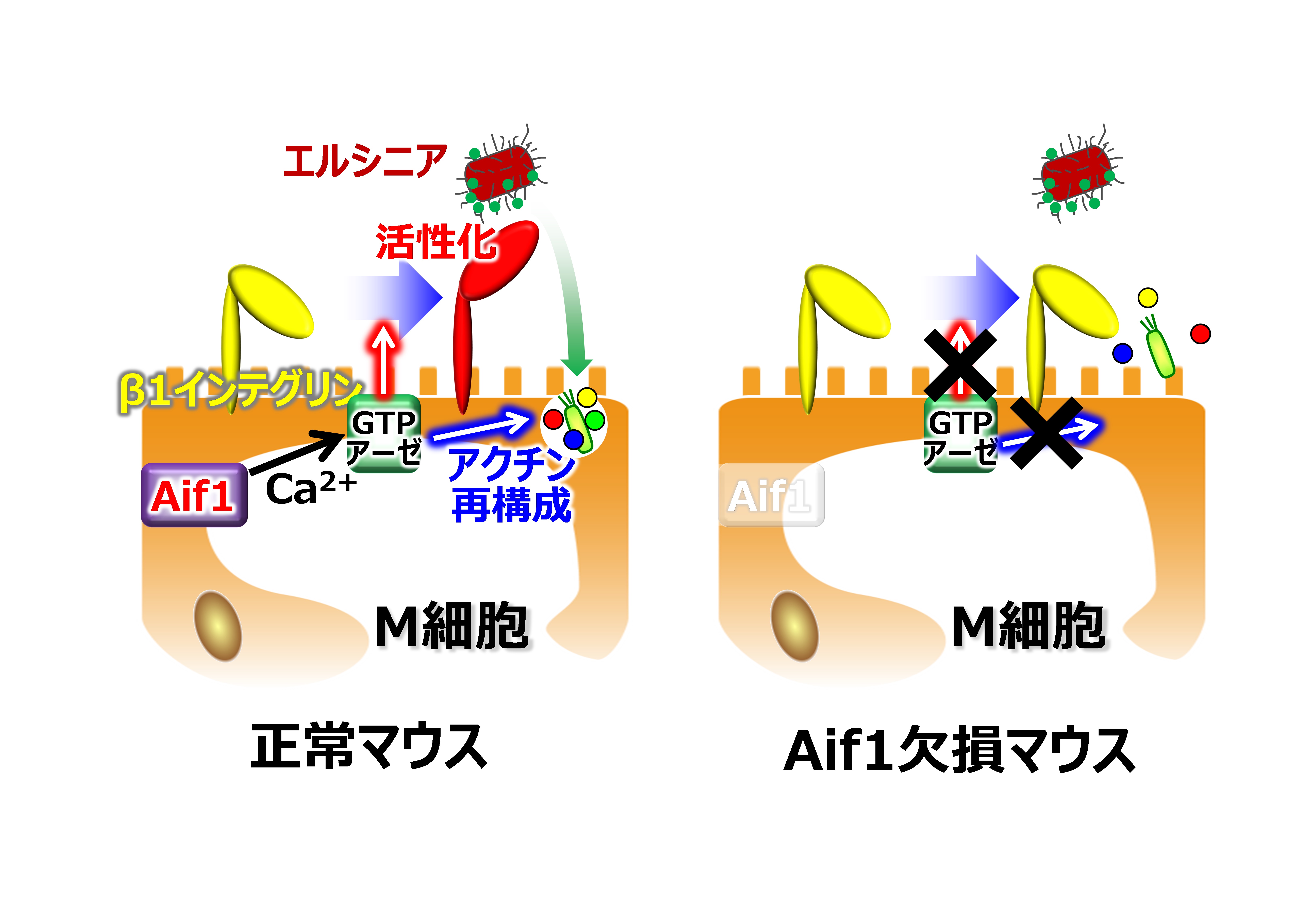
Figure 3.
To learn more about this research, please view the full research report entitled “ Allograft inflammatory factor 1 is a regulator of transcytosis in M cells ” at this page of the Nature Communications website.
Related link
