High-resolution pH Imaging Elucidates Energy Mechanisms in Creating Bacterial Flagella
Osaka University researchers develop methods to detect pH in vivo, and elucidate phenomena driving protein export in biological activities
Bacterial cellular membranes protrude to form flagella, which are composed of up to about 30 distinct proteins and are essential for motility. The bacterial flagellar type III export apparatuses utilize energy derived from ATP hydrolysis and proton motive force (PMF) across the cell membrane to translocate flagellar proteins across the membrane. Previous studies have demonstrated that the flagellar transmembrane export gates play crucial roles in energy transduction to unfold and transport flagellar proteins for construction of the flagella in a PMF-powered manner. However, the energy transduction mechanisms have not been completely understood.
Now, by developing a high-resolution imaging system with high spatial and pH resolutions, Osaka University researchers have successfully correlated pH levels with intracellular localization. They used fluorescence-based optical microscopy with a dual-wavelength illumination system and an electron-multiplying charge-coupled device camera to detect the presence of a fluorescent protein probe pHluorin. The ratiometric pH probe pHluorin was modified with an M153R mutation to enhance brightness and stability and used to measure the microenvironmental pH surrounding the probe between 5.5 and 8.5 at the emission intensity ratio of 410/470. The objective was to characterize the role played by ATP and protons (H + ) in flagellar protein export in Salmonella . The research was recently reported in mBio .
Interestingly, the researchers noted there were small but significant differences in intracellular pH. “We detected highest pH levels at the plasma membranes, intermediate levels at the export gates and lowest levels in the remaining cytoplasm. Accordingly, we propose that the export apparatus uses both ATP hydrolysis as well as H + differentials to achieve protein export,” study corresponding author Keiichi Namba says.
Importantly, this study proposes that the FliH 12 FliI 6 FliJ cytoplasmic ATPase complex and the export gate work in concert to drive H + flow inward via the gate, mediated by Flil ATPase, as well as initiate protein export outward. It thereby establishes that pH imaging provides a realistic window into elucidating cellular responses and signaling in the context of energy transfer.
This study represents an important step toward describing the mechanisms underlying the complex energy transduction process. Since the maintenance of intracellular pH homeostasis is vital for functional cellular responses, the sensitive and accurate imaging of pH in living organisms has gained importance in medical science and in pharmacology.
Namba believes this methodology is a significant advancement: “Our findings suggest that pHluorin probes represent an exciting class of pH probes with multi-functional applications in diverse areas of biology and pharmaceutical sciences.”
Abstract
Protons are utilized for various biological activities such as energy transduction and cell signaling. For construction of the bacterial flagellum, a type III export apparatus utilizes ATP and proton motive force to drive flagellar protein export, but the energy transduction mechanism remains unclear. Here, we have developed a high-resolution pH imaging system to measure local pH differences within living Salmonella enterica cells, especially in close proximity to the cytoplasmic membrane and the export apparatus. The local pH near the membrane was ca. 0.2 pH unit higher than the bulk cytoplasmic pH. However, the local pH near the export apparatus was ca. 0.1 pH unit lower than that near the membrane. This drop of local pH depended on the activities of both transmembrane export components and FliI ATPase. We propose that the export apparatus acts as an H + /protein antiporter to couple ATP hydrolysis with H + flow to drive protein export.

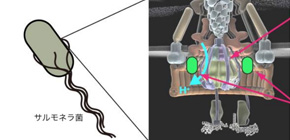
Figure 1: Bacterial flagellar protein export apparatus. The flagellar protein export apparatus is embedded in the cytoplasmic membrane and is required for construction of the bacterial flagellum as a motility organelle. To monitor the local pH around the export apparatus, a pH-sensitive fluorescent protein was fused to an inner face of the flagellar motor.

Figure 2: Local pH near the export apparatus. In the absence of ATPase, the local pH value is higher than that of wild type.
To learn more about this research, please view the full research report entitled " High-Resolution pH Imaging of Living Bacterial Cells To Detect Local pH Differences " at this page of the mBio website.
Related links
