Calcium Aids Chromosome Condensation Prior to Cell Division
Research reveals role for calcium ions in chromosome condensation during mitosis
Before cell division when a parent cell divides into two daughter cells, each with an identical set of genetic information, replicated chromosomes become condensed and align along the spindle in the middle of the cell. This is the metaphase stage of mitosis. Chromosomes are made up of strands of DNA wrapped around histones, forming nucleosomes that resemble beads on a string. The chromosome structure is maintained by histone changes, chromosome scaffold proteins, and positively charged ions (cations). However, removal of scaffold proteins has no effect on chromosome condensation, suggesting that another factor causes the organization of metaphase chromosomes. Now, work led by Osaka University has revealed that calcium ions (Ca 2+ ) play a crucial role in chromatin fiber compaction. The study was reported in Scientific Reports .
Calcium is a universal second messenger with many functions, including progression of the cell cycle. During mitosis, both Ca 2+ and magnesium ions (Mg 2+ ) are released from storage organelles to bind chromatin, though 6–8-fold more Ca 2+ is bound than Mg 2+ suggesting that it has a more important role. A research team led by Hideaki Takata, Kiichi Fukui and Rinyaporn Phengchat at Osaka University first confirmed that Ca 2+ is necessary to prevent chromosome misalignment during mitosis, then used an imaging assessment of molecular interactions based on fluorescence decay (FLIM–FRET) to show that chromosomes were less compact in the absence of intracellular Ca 2+ .
This same imaging process also revealed that changes in Ca 2+ levels of living HeLa cells altered the extent of chromosome compaction, with the re-addition of Ca 2+ causing chromosome compaction. Ca 2+ therefore appears to control the transition between condensation and decondensation. “Although previous studies have demonstrated nucleosome packaging using isolated chromatin” study first author Rinyaporn Phengchat says, “such findings should be confirmed in vivo as we have done using the sensitive FLIM–FRET technique which provides a high level of spatial resolution.”
The team then used scanning electron microscopy to visualize chromosome structures at very high resolution. “Ca 2+ has a concentration-dependent effect on chromosome structure,” corresponding author Hideaki Takata explains, “causing chromosomes to change from expanded fibrous structures to compact globular structures with increasing levels of Ca 2+ .”
The researchers propose that, in the absence of Ca 2+ , the negatively charged DNA is less well neutralized, preventing it from condensing and delaying entry into prometaphase of the cell cycle. “We think that a lack of Ca 2+ disturbs the organization of chromosomes at the spindle, causing them to misalign,” corresponding author Kiichi Fukui says. “Conversely, the normal rising levels of Ca 2+ during mitosis promote chromosome condensation after breakdown of the nuclear envelope.”
Abstract
Chromosome condensation is essential for the faithful transmission of genetic information to daughter cells during cell division. The depletion of chromosome scaffold proteins does not prevent chromosome condensation despite structural defects. This suggests that other factors contribute to condensation. Here we investigated the contribution of divalent cations, particularly Ca 2+ , to chromosome condensation in vitro and in vivo. Ca 2+ depletion caused defects in proper mitotic progression, particularly in chromosome condensation after the breakdown of the nuclear envelope. Fluorescence lifetime imaging microscopy-Förster resonance energy transfer and electron microscopy demonstrated that chromosome condensation is influenced by Ca 2+ . Chromosomes had compact globular structures when exposed to Ca 2+ and expanded fibrous structures without Ca 2+ . Therefore, we have clearly demonstrated a role for Ca 2+ in the compaction of chromatin fibres.

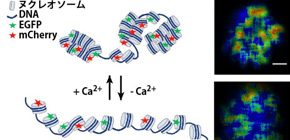
Figure 1. The effects of Ca 2+ on chromatin condensation revealed by the measurement of the change in fluorescence life time. Nucleosome was labelled by EGFP and mCharry and it enables us to monitor chromatin condensation status by measuring the change in the fluorescence life time. Higher Ca 2+ concentration induces shorter fluorescence life time indicating chromatin condensation (upper panel). In contrast, lower Ca 2+ induces longer life time indicating chromatin decondensation (lower panel). Bar, 100 nm.

Figure 2. Schematic illustration to monitor chromatin condensation using FLIM-FRET. FRET occurs when two fluorescent proteins, EGFP and mCherry locate closely each other. The FRET reduces the life time of GFP. If nucleosomes are labelled by EGFP and mCherry, chromatin decondensation causes the increase in the dissociation of EGFP and mCherry, and the life time of EGFP becomes longer. In contrast, chromatin condensation causes the increase in the access of EGFP and mCherry, and the life time of EGFP becomes shorter. The life time can be measured using FLIM.
To learn more about this research, please view the full research report entitled “ Calcium ions function as a booster of chromosome condensation ” at this page of the Scientific Reports website.
Related link
