Resonant Inelastic X-ray Scattering Technique Developed
Technique for Fully Examining Electronic States in Catalytic Activity
Japan Atomic Energy Agency, Osaka University, and Daihatsu Motor Co., Ltd., succeeded in developing a technique for fully examining electronic states in catalytic activity.
- Japan Atomic Energy Agency -- Ignace Jarrige , Researcher (currently, Researcher, Brookhaven National Laboratory)
- Graduate School of Engineering, Osaka University -- KASAI Hideaki (Professor)
Abstract
Self-regenerating automotive catalysts owe their remarkable performance to the repeated motion of the precious metal atoms in and out of the perovskite lattice under fluctuating oxidizing and reducing conditions, preventing coalescence of the metal nanoparticles. Here we use resonant inelastic X-ray scattering to characterize the occupied and unoccupied Pt 5d states in two self-regenerating Pt-perovskite catalysts, CaTi0.95Pt0.05O3 and CaZr0.95Pt0.05O3. Upon reduction, the element and symmetry-specific charge excitation spectra reveal a sizable hybridization between the Pt 5d and the Ti 3d or Zr 4d states at the interface between the nanoparticles and the perovskite, which involves the occupied states and is thus invisible in X-ray absorption spectra. A correlation is found between the strength of this d-band hybridization and the proportion of Pt nanoparticles that remain buried below the surface during reduction, indicating that the motion of the Pt atoms toward the surface is hindered by this hybridization specifically, rather than by the Pt–O bonding. These results provide direct evidence that the strength of the metal–metal d-band hybridization plays a pivotal role in determining the efficiency of self-regeneration in perovskite catalysts.
Catalytic reactions are an important part of everyday life. Gas exhausts from car engines contain harmful compounds like poisonous carbon monoxide, and nitrogen oxides which cause smog and acid rain. Modern automobiles are equipped with catalytic converters which, thanks to their purifying ability, can convert these into harmless compounds before leaving the exhaust system. The catalysts used in a catalytic converter are made up of precious metals, which are scarce relative to most other elements, which is why scientists are in pursuit of new, efficient catalysts that can work with smaller amounts of precious metals.
This is where the so-called “intelligent catalysts,” developed by Daihatsu Motor Co., Ltd., have succeeded. Intelligent catalysts rely on the cyclical motion of precious metal nanoparticles in and out of the substrate – a mineral powder into which precious metals are injected – during the reduction-oxidation cycles of the engine. This constant motion prevents the nanoparticles from collecting into groups when exposed to heat during vehicle use, achieving high efficiency and durability with small quantities of precious metal.
Intelligent catalysts are now successfully installed in several million vehicles in Japan. While most of the research in the field so far has focused on the structure of the catalysts, some recent research efforts have pointed to the role played by changes in the electronic structure during catalytic reactions. Because catalytic reactions occur through complex exchanges of electrons between the reactant molecules, the catalyst, and the catalyst substrate, it has become essential to use a measurement technique that can follow these changes in the electronic structure as they occur within the catalytic reaction.
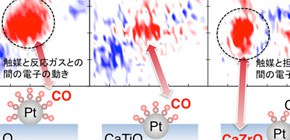
To better understand these electronic exchanges, a team of scientists from the Japan Atomic Energy Agency and Osaka University in collaboration with Daihatsu Motor Co., Ltd. measured two types of platinum-based automotive catalysts – conventional and intelligent. They found that while the conventional catalyst only showed an electron transfer between the platinum nanoparticles and the reactant carbon monoxide molecule, there is an additional electron transfer between the platinum nanoparticles and the metal within the substrate in the intelligent catalysts, indicating that the platinum nanoparticles still ‘feel’ the metal atoms inside the substrate even when they are out of the substrate on the surface during reduction.
Platinum and zirconium, the metal inside the intelligent catalyst substrate, both have extended electronic orbitals – paths along which individual electrons circle the atoms within the metals – which can overlap even when the atoms are not in close physical proximity. This special overlap between the atom orbitals can be seen as a ‘rubber band’ pulling the platinum atoms back into the substrate after they have been pushed out during reduction. The researchers think this ‘electronic rubber band’ effect is at the heart of the efficiency and durability of the intelligent catalysts. Further, comparing two intelligent catalysts with different amplitudes of orbital overlap between the platinum nanoparticles and the substrate, they found that a moderate amplitude (i.e. a rubber band of moderate strength) is best for the efficiency of the catalyst.
To determine how the electronic rubber band effect works, they used resonant inelastic x-ray scattering (RIXS), a state-of-the-art spectroscopy technique that relies on a highly precise detection of x-rays scattered off of a sample. RIXS has recently emerged as a powerful probe of electron transfer processes, and as such, has been very instrumental in characterizing the electronic structure of superconducting materials. While becoming a highly demanded tool in condensed matter physics, applications of RIXS to chemistry and catalysis in particular have remained scarce, despite obvious benefits such as the use of high-energy x-rays that make experiments in constrained environments like in-situ cells possible, and the ability to select one particular element within a sample (for instance, the precious metal in a catalyst). In this study, the team put these benefits to good use by performing RIXS measurements on automotive catalysts under working conditions (oxidizing and reductive atmospheres) in in-situ cells. The experiments were performed at the RIXS endstation of the JAEA beamline BL11XU at the Japanese synchrotron SPring-8.
FIGURE: RIXS intensity planes showing a strong interaction between the Pt and CO valence electrons for the conventional catalyst Pt/Al2O3. For the intelligent catalysts, a weaker Pt-CO interaction is observed for Pt/CaTiO3, while Pt/CaZrO3 shows a strong interaction between Pt and the substrate.
Glossary
Resonant Inelastic X-ray Scattering : With resonant inelastic x-ray scattering, the energy of the incident x-rays is tuned to match an absorption edge of the element of interest inside the sample. The energy of the x-rays scattered off from the sample is measured, hence the energy transferred from the incoming x-rays to the sample, corresponding to the energy of electronic excitations between the occupied and unoccupied valence states, can be inferred. These electronic excitations are fingerprints of the valence electronic orbitals of the studied element, and bear important information about orbital mixing and chemical bonding. Intelligent Catalysts : The high operating temperatures of catalytic converters cause the precious metal nanoparticles to agglomerate, reducing the total surface area and resulting in a decrease of their purification performance over mileage. Intelligent catalysts rely on the cyclical motion of precious metal atoms in and out of a perovskite lattice during the natural fluctuations of the engine atmosphere, reductive and oxidizing. Thanks to this so-called self-regeneration process, coalescence of the precious metal atoms on the surface of the perovskite substrate is avoided, and a high purification performance can be maintained during aging. Beamline BL11XU at SPring-8 : BL11XU is one of the 4 beamlines operated by the Japanese Atomic Energy Agency at SPring-8. It is equipped with a spectrometer dedicated to hard x-ray resonant inelastic x-ray scattering, shown in the photo.
To learn more about this research, please view the full research report entitled " Toward Optimizing the Performance of Self-Regenerating Pt-Based Perovskite Catalysts " at this page of the ACS Catalysis website.
For more information, please contact:
Japan Atomic Energy Agency
Ignace Jarrige (present address: Brookhaven National Laboratory)
Email: jarrige@bnl.gov
Kenji Ishii
Email: kenji@spring8.or.jp
Related Link
