Successful mimicking of ocular development with human iPS cells and generation of corneal epithelium
Prospects for development of new techniques in regenerative medicine to treat intractable corneal disease
- Development of the world’s first 2D culture system* 1 to generate an eye from human iPS cells
- Use of this culture system to generate functional corneal epithelium from concentric rings of cells (an SEAM) for the first time
- Using iPS cells to reconstruct the corneal epithelium in humans and to facilitate the development of techniques to reconstruct various portions of the eye
Overview
Research groups of Professor Kohji Nishida of the Department of Ophthalmology, Endowed Associate Professor Ryuhei Hayashi of the Department of Stem Cells and Applied Medicine, Osaka University Graduate School of Medicine, and others developed a 2D culture system to promote cell-autonomous* 2 differentiation of human iPS cells. This system mimics the development of the whole eye (Fig. 1). In the past, studies have only described techniques to generate the posterior portion of the eye (the retina, the pigmented epithelium of the retina, etc.). In contrast, the current study is the first to ever describe a technique that can generate both the anterior portion (the cornea, lens, etc.) and the posterior portion of the eye (the retina, the pigmented epithelium of the retina, etc.) at the same time.
Donor cornea is used to perform a corneal transplantation to treat serious diseases of the corneal epithelium that lead to blindness, but this approach is hampered by rejection and a shortage of donors. In the past, there were no techniques to induce human iPS cells to differentiate into corneal epithelial cells and to then isolate those cells to create functional corneal epithelium.
The culture system developed in this study can use human iPS cells to generate a 2-dimensional structure (a self-formed ectodermal autonomous multi-zone (SEAM)) consisting of 4 concentric zones of cells. Major groups of cells that comprise the eye during development (e.g. corneal epithelium, the retina, and the epithelium of the lens) are produced at specific locations in the SEAM. The current study successfully isolated corneal epithelial progenitor cells from the 3rd zone of the SEAM, and this study also successfully generated functional corneal epithelium. Corneal epithelium produced from human iPS cells was transplanted in an animal model, where that corneal epithelium was therapeutically effective (Fig. 2).
Results of this study should greatly help to facilitate reconstruction of the corneal epithelium with iPS cells in humans. In addition, the SEAM has the potential to facilitate the development of techniques to reconstruct the cornea as well as other portions of the eye.
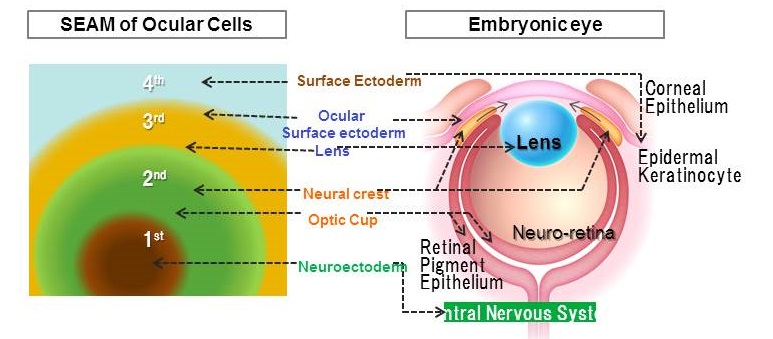
Figure 1. A self-formed ectodermal autonomous multi-zone (SEAM)
(A) A SEAM generated from human iPS cells consists of 4 concentric zones that mimic the natural development of the whole eye.
(B) Major groups of cells that comprise the eye during development (e.g. corneal epithelium, the retina, and the epithelium of the lens) are produced at specific locations in the SEAM.
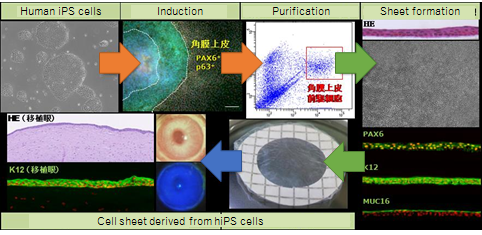
Figure 2. Generation of functional corneal epithelium from a SEAM
A SEAM was generated from human iPS cells and corneal epithelial progenitor cells were isolated from the SEAM. Stratified human corneal epithelium was then generated. This corneal epithelium from human iPS cells was then transplanted in an animal, indicating that the corneal epithelium was therapeutically effective.
Background for the study
When corneal epithelial stem cells are lost due to trauma or diseases, the surrounding conjunctival tissue invades the cornea and blood vessels. The cornea then loses its transparency, leading to blindness. Corneal allo-transplantation has been performed with an allograft donor cornea (from an eyebanked eye donated by someone else) to treat serious diseases of the corneal epithelium, but treatment outcomes are limited because of rejection. A shortage of donors is also a problem. To resolve these problems, Professor Nishida and colleagues previously developed a technique known as cultivated oral mucosal epithelial cell transplantation (COMET) and they used this technique in clinical settings. COMET reconstructs the corneal epithelium by transplanting oral mucosal epithelial cells as replacements, i.e. transplantation of sheets of cultivated autologous oral mucosal epithelial cells. This technique has proven superior to conventional corneal allo-transplantation. However, long-term observation has revealed that COMET has limited effectiveness since phenomena occur due to differences in the properties of the cornea and the oral mucosa. As an example, vascular invasion of the cornea occurs, sometimes causing the cornea to cloud.
If corneal epithelial cells could be produced from a patient’s own cells, then these problems could be resolved. Human iPS cells are pluripotent and can differentiate into any given cell lineage. These cells can also serve as a source of autologous cells that can avoid immunological rejection, so iPS cells could be used in regenerative medicine to treat intractable corneal disease. In the past, there were no techniques to induce human iPS cells to differentiate into corneal epithelial cells and to then isolate those cells to create functional corneal epithelium. Thus, Professor Nishida and colleagues worked to develop a technique to use human iPS cells to obtain corneal epithelial cells that could be used to reconstruct the cornea.
The effect of the study’s results on society (the significance of the study’s results)
Results of this study should greatly help to facilitate reconstruction of the corneal epithelium with iPS cells in humans. The significance of this study is that the mechanism for development of the human eye was unclear, but the SEAM allows detailed analysis of this mechanism. In addition, the SEAM has the potential to facilitate the development of techniques to reconstruct the cornea as well as other portions of the eye.
Note
The results of this study are scheduled to be featured in the online version of Nature at 6:00 PM (UK time) on Wednesday, March 9, 2016.
Title of the paper: Co-ordinated ocular development from hiPSCs and recovery of corneal function
Authors: Ryuhei Hayashi, Yuki Ishikawa, Yuzuru Sasamoto, Ryosuke Katori, Naoki Nomura, Tatsuya Ichikawa, Saori Araki, Takeshi Soma, Satoshi Kawasaki, Kiyotoshi Sekiguchi, Andrew J. Quantock, Motokazu Tsujikawa, and Kohji Nishida
Also, this study was implemented mainly under the support of “Research Center Network for Realization of Regenerative Medicine - Highway Program for Realization of Regenerative Medicine” sponsored by the Ministry of Education, Culture, Sports, Science and Technology, the Japan Science and Technology (JST), and the Japan Agency for Medical Research and Development (AMED).
Explanation of terms
*1 2D culture system: A monolayer of cells cultured in a culture dish is referred to as a 2D culture. In contrast, cells cultured at a certain thickness are referred to as a 3D culture.
*2 Cell-autonomous: Cell-autonomous means that cells do something (e.g. differentiate or proliferate) themselves without help from other cells or factors.
Related links
