First transplantation of iPS cells-derived corneal epithelial cell sheets
A group of researchers led by Prof. NISHIDA Kohji of Osaka University transplanted iPS cells-derived corneal epithelial cell sheets into a patient with corneal disorders in July 2019.
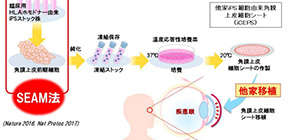
This is the first clinical study of cornea regeneration using iPS to examine the safety and effects of sheet-shaped corneal epithelial cells, which are cultivated by inducing corneal epithelial cells using iPS cells from others (provided by the Center for iPS Cell Research and Application (CiRA), Kyoto University), with their own method. The status of the patient, who was discharged from hospital on August 23, 2019, will be continually monitored.
Corneal epithelial stem cell deficiency caused by damage to the corneal epithelium has challenges, such as donor shortage and rejection in recipients of transplants using donor corneas. In order to ultimately solve these challenges, the research group has advanced the development of regenerative therapies using hiPS-derived corneal cells.
After gaining approval for the world’s first clinical study for the transplantation of human cornea tissues differentiated from human induced pluripotent stem (hiPS) cells into patients with corneal disorders from the Ministry of Health, Labour and Welfare in March 2019, the group started the clinical study.
In this study, four patients with severe corneal epithelial stem cell deficiency will undergo transplantation of sheets of iPS cells from donors. In the first two cases, two patients will undergo transplantation of sheets of iPS cells from donors who do not share the same human leukocyte antigen-3 (HLA-3) with these two patients with concomitant administration of immunosuppressing drugs. After that, the group will perform the interim assessment of these two cases.
Then, the team will perform HLA typing to examine a donor-recipient HLA match for the remaining two patients and decide if they will administer immunosuppressing drugs to these patients. They will follow these four patients for a year after transplantation.
The primary endpoint of this clinical study is safety, for which the team will collect and evaluate adverse effects observed during the study. For secondary endpoints, they will evaluate the efficacy of the transplant, including improvement of the conditions of corneal epithelial stem cell deficiency and degree of vision recovery.
Currently, the researchers are monitoring the first transplant recipient of iPS cell-derived corneal epithelial cell sheets and plan to perform transplant of iPS cell-derived corneal epithelial cell sheets to the second patient within this year. They will perform this first-in-human (FIH) clinical study on corneal epithelial cell sheets from hiPSCs in order to develop it into clinical trials and eventually into standard medical treatment. Because this method can overcome challenges in existing therapies, especially donor shortage and transplant rejection, it will improve the vision of many patients who are in danger of blindness due to corneal disorders throughout the world.
Figure 1
Related links
- Department of Ophthalmology, Graduate School of Medicine, Osaka University (link in Japanese)
