Critical Role of Interleukin-6/Interleukin-21 signaling in Pulmonary Arterial Hypertension Clarified
May lead to the development of novel treatment for pulmonary arterial hypertension
A group of researchers from Osaka University, National Cerebral and Cardiovascular Center, The University of Tokyo, and Hokkaido University demonstrated the critical roles of inflammatory cytokines, interleukin-6 and interleukin-21, in the pathogenesis of pulmonary arterial hypertension (PAH), one of refractory cardiovascular diseases. Their research also clarified that interleukin-6/interleukin-21-signaling axis promotes PAH in association with M2 macrophage polarization.
Internal Medicine, Department of Cardiovascular Medicine, Graduate School of Medicine, Osaka University: NAKAOKA Yoshikazu (Assistant Professor), HASHIMOTO-KATAOKA Takahiro (Graduate Student), SAKATA Yasushi (Professor)
Inflammation and autoimmunity have been recognized as critical contributors to the pathogenesis of PAH. Among the inflammatory cytokines, interleukin-6 (IL-6) has been reported to be critically involved in the pathogenesis of PAH; however, the molecular mechanism of the development of PAH mediated by IL-6 has been elusive. This research group identified interleukin-21 (IL-21) as a key downstream target of IL-6 signaling in PAH.
In this research, the researchers used hypoxia-induced pulmonary hypertension (HPH) mouse model and found that the expression of IL-6 was strongly in the intima and medial layers of the arterioles and small arteries of the lungs after exposure to hypoxia. The researchers found that IL-6 blockade by anti-interleukin-6 receptor antibody (anti-IL-6R Ab) ameliorated HPH (Figure 1A).
Next, they focused on Th17 cells and Th17 cytokines, since the differentiation of Th17 cells depends on IL-6 and TGF- β . Treatment with anti-IL-6R Ab prevented the hypoxia-induced accumulation of Th17 cells and the upregulation of interleukin-17 (IL-17) and IL-21 genes, one of the signature genes for Th17 cells in the lungs of mice after hypoxia exposure, compared with control antibody treatment (Figure 1B).
In further research, they found that IL-21 receptor knockout (IL-21RKO) mice showed resistance to HPH, although IL-17 blockade with anti-IL-17 neutralizing Ab had no significant effect on HPH (Figure 2). Taken together, these findings suggest that IL-21 is critical in the pathogenesis of PAH downstream of IL-6 in mice. The researchers found both IL-6 and IL-21 were required for the accumulation of M2 macrophages in the lungs of HPH mice (Figure 2). Consistent with these findings, significantly enhanced expressions of IL-21 and M2 macrophage markers were detected in the lungs of IPAH patients who underwent lung transplantation (Figure 3).
Their findings suggest that IL-21 promotes PAH in association with M2 macrophage polarization, downstream of IL-6-signaling. IL-6/IL-21-signaling axis might be a potential therapeutic target for PAH.
Hypoxia induces the upregulation of IL-6 in the pulmonary arterial endothelial cells (orange) and pulmonary arterial smooth muscle cells (PASMCs; yellow). IL-6 promotes the differentiation of Th17 cells from naïve T cells presumably in cooperation with TGF- β in the lung. IL-17, mainly derived from Th17 cells, is partly responsible for the production of IL-21 in these cells. IL-21 promotes macrophage skewing toward the M2 phenotype in the alveolar macrophages. M2 macrophages positively regulate the hypoxia-induced proliferation of PASMCs by releasing humoral factors such as CXCL12. Collectively, IL-21 plays a critical role in the pathogenesis of PAH in concert with M2 macrophage polarization downstream of IL-6.
Abstract
Interleukin-6 (IL-6) is a multifunctional proinflammatory cytokine that is elevated in the serum of pulmonary arterial hypertension (PAH) patients and can predict the survival of idiopathic (I)PAH patients. Previous animal experiments and clinical human studies indicate that IL-6 is important in PAH; however, the molecular mechanisms of IL-6-mediated pathogenesis of PAH have been elusive. Here we identified IL-21 as a novel downstream target of IL-6-signaling in PAH. First, we found that IL-6 blockade by the monoclonal anti-IL-6 receptor antibody, MR16-1, ameliorated hypoxia-induced pulmonary hypertension (HPH) and prevented the hypoxia-induced accumulation of Th17 cells and M2 macrophages in the lungs. Consistently, the expression levels of IL-17 and IL-21 genes, one of the signature genes for Th17 cells, were significantly upregulated after hypoxia exposure in the lungs of mice treated with control antibody, but not in those treated with MR16-1. Whereas IL-17 blockade with an anti-IL-17A neutralizing antibody had no effect on HPH, IL-21 receptor-deficient mice were resistant to HPH and exhibited no significant accumulation of M2 macrophages in the lungs. In accordance with these findings, IL-21 promoted the polarization of primary alveolar macrophages toward the M2 phenotype. Of note, significantly enhanced expressions of IL-21 and M2 macrophage markers were detected in the lungs of IPAH patients who underwent lung transplantation. Collectively, these findings suggest that IL-21 promotes PAH in association with M2 macrophage polarization, downstream of IL-6-signaling. IL-6/IL-21-signaling axis may be a novel potential target for treating PAH.
Figure 1: The effect of anti-interleukin-6 receptor (anti-IL-6R) antibody (Ab) on the hypoxia-induced pulmonary hypertension (HPH) mice
A. Hypoxia-induced thickening of the pulmonary vasculatures was observed in the lungs of the control mice treated with control Ab, but not in those treated with anti-IL-6R Ab.
B. IL-21 was significantly upregulated in the lungs of the hypoxia-induced PAH model mice treated with control Ab, but not in those treated with anti-IL-6R Ab.
Figure 2: Interleukin-21 receptor knockout (IL-21RKO) mice show resistance to hypoxia-induced pulmonary hypertension (HPH)
In wild-type (WT) mice, hypoxia exposure induces accumulation of M2 macrophages in the lungs (upper panel) and increased proliferation of pulmonary artery smooth muscle cells (PASMCs) (middle panel) at early phase, and finally induces thickening of the medial wall thickening of pulmonary arterial vasculatures at late phase (lower panel). On the other hand, accumulation of M2 macrophages in the lungs (upper panel), increased proliferation of pulmonary artery smooth muscle cells (PASMCs) (middle panel) at early phase, and medial wall thickening of pulmonary arterial walls at late phase (lower panel) were inhibited in IL-21RKO mice.
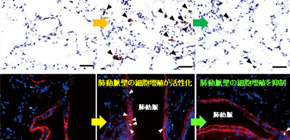
Figure 3: Enhanced expression of IL-21 and the marker of M2 macrophages in the lungs of idiopathic pulmonary arterial hypertension (PAH) patients
An excessive infiltration of IL-21-positive cells was detected in the adventitia, obliterative intimal proliferative lesions, and plexiform lesions of the remodeled pulmonary arteries (A). Strong infiltration of M2 macrophages were detected in all layers of the remodeled pulmonary arteries and alveolar areas examined in the lungs from IPAH patients, but not in the tissue from control patients (B).
To learn more about this research, please view the full research report entitled " Interleukin-6/interleukin-21-signaling axis is critical in the pathogenesis of pulmonary arterial hypertension " at this page of the Proceedings of the National Academy of Sciences of the United States of America website.
Related Links
