Mechanisms Behind the Botulinum Toxin Pathogenesis Clarified
The botulinum neurotoxin complex is known to cause fatal food poisoning
A group of researchers led by FUJINAGA Yukako (Specially Appointed Professor) and MATSUMURA Takuhiro (Specially Appointed Assistant Professor) at the Research Institute for Microbial Diseases, Osaka University, and OHNO Hiroshi (Group Director, RIKEN) clarified how botulinus toxin in the intestinal tract invaded the body. The botulinum neurotoxin complex is known to cause fatal food poisoning. This group's research has clarified that hemagglutinin (HA), one of the nontoxic components of a botulinus toxin complex, binds to GP2, a membrane protein expressed on M cells in the intestinal epithelium, by which botulinus toxin invades the body through M cells.
This group's achievement could possibly contribute to the development of preventive and therapeutic methods of botulism poisoning. The application of this research may also lead to the development of new transmucosal drug delivery systems and transmucosal vaccines by making use of the invasion mechanism of botulinus toxin.
Abstract
To cause food-borne botulism, botulinum neurotoxin (BoNT) in the gastrointestinal lumen must traverse the intestinal epithelial barrier. However, the mechanism by which BoNT crosses the intestinal epithelial barrier remains unclear. BoNTs are produced along with one or more non-toxic components, with which they form progenitor toxin complexes (PTCs). Here we show that serotype A1 L-PTC, which has high oral toxicity and makes the predominant contribution to causing illness, breaches the intestinal epithelial barrier from microfold (M) cells via an interaction between haemagglutinin (HA), one of the non-toxic components, and glycoprotein 2(GP2). HA strongly binds to GP2 expressed on M cells, which do not have thick mucus layers. Susceptibility to orally administered L-PTC is dramatically reduced in M-cell-depleted mice and GP2-deficient (Gp2_/_) mice. Our finding provides the basis for the development of novel antitoxin therapeutics and delivery systems for oral biologics.
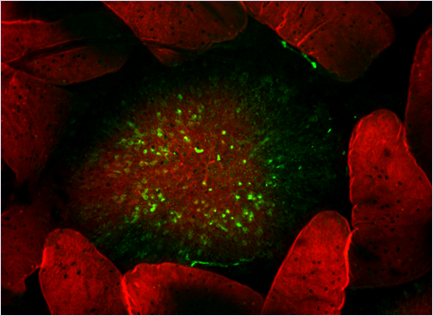
Figure 1:
Botulinum toxin complex (green) is taken up by Peyer's patch M cells.
Alexa Fluor 488–labeled botulinum toxin complex (type A, L-toxin) was injected into ligated mouse intestinal loops containing Peyer’s patches, and incubated for 2 h. Whole-mount intestinal villous epithelium (VE) regions and follicle-associated epithelium (FAE) regions were stained with Alexa Fluor 568–labeled phalloidin (red)
Figure 2:
Botulinum neurotoxin complex (type A) breaches the intestinal epithelial barrier from microfold (M) cells via an interaction between hemagglutinin (HA), one of the nontoxic components, and glycoprotein 2(GP2).
To learn more about this research, please view the full research report entitled "Botulinum toxin A exploits intestinal M cells to enter the host and exert neurotoxicity" at this page of the Nature communications website.
Related Link
