
Development of novel technique for growing protein crystals in totally gellified hydrogel
Synopsis
SUGIYAMA Shigeru , Specially Appointed Associate Professor, Graduate School of Science, Osaka University, MATSUMURA Hiroyoshi , Associate Professor, Graduate School of Engineering, Osaka University, and ADACHI Hiroaki , President, Sosho Inc., succeeded in developing a new technique --patent pending-- for growing protein crystals. Furthermore, they have clarified that protein crystals produced by this method are able to avoid damage by osmotic shock.
The research team expects that this technique will accelerate the discovery of proteins causing diseases and of crystals of complexes for lead compounds that can be dissolved only in organic solvents. This technique promises to be applicable to screening for drug discovery, a process in which new drug discoveries are obtained through screening large numbers of lead compounds.
This research achievement was published in the American chemical magazine, Journal of the American Chemical Society on April 4, 2012. This research paper was also introduced as one of Spotlights on Recent JACS Publications on April 11, 2012.
Background
More than 100,000 proteins work in the body. A variety of proteins such as keratin in hair and nails and collagen in skin and bones are essential for life processes. Proteins are basic building blocks of our bodies. Each protein has a very complex and specific shape. These shapes greatly influence life support processes. When we are healthy, proteins work normally; however, they may exhibit abnormal behavior when we are sick.
When we are ill, a drug we take may be able to bind with "pits" in an abnormal protein, suppressing its action. As a result, such a drug may help cure the illness. One can explain this phenomenon with the analogy of a key and keyhole. The pit in a protein is the keyhole and the drug is the key. In other words, if we can find a key for the keyhole, we have found and can develop that key, that drug.
In order to understand the details of these keyholes accurately, it's necessary to visualize the structure of the combined protein and drug. The technology needed for creating such proteins is protein crystallization. With current technology, it's possible to grow protein crystals by binding protein molecules and irradiating them with X-rays to reveal their stereoscopic structure.
However, protein crystals are soft and fragile like tofu making them very difficult to handle. Furthermore, in developing new drugs, in order to grow crystals of complexes of the target protein and lead compounds, it's necessary to soak protein crystals in solvents containing various compounds. However, chemical compounds are dissolved in high-concentration organic solvents or saline solutions and these solutions damage the proteins by osmotic shock during soaking. Heretofore, this has been a major problem.
Findings
In order to solve this problem, specially appointed associate professor Sugiyama et al at the Graduate School of Science worked on the development of new crystallization techniques for growing protein crystals in a completely gellified [solidified] gel solution. (Figure 1)
Typically protein crystals are grown in liquid solutions so Sugiyama et al had to develop new and original techniques for growing them in a completely gellified gel. The crystals grown by their technique are surrounded by the gellified gel, which acts as a protector, making it possible for these crystals to be handled without touching them directly. (Figure 2)
Via this method, high quality protein crystals were easily obtained. The group also succeeded in obtaining accurate and reproducible X-ray diffraction analysis data. The group has demonstrated that protein crystals produced by using this new technique avoid damage by osmotic shock during soaking in high-concentration organic solvents or saline solutions. (Figure 3)
In order to discover why such crystals avoided osmotic shock damage, the group measured the strength of the crystals using Vickers hardness meter. (Figure 4) It was found that the crystals grown in gellified gel had far greater mechanical hardness than those grown by conventional methods. (Figure 5)
Under an optical microscope called LCM-DIM (laser confocal microscopy combined with differential interference contrast microscopy), the protein crystals grown in gellified gel exhibited heretofore never observed hexagonal etch pits on the surface. (Figure 6) These pits are thought to be part of the gel incorporated into the crystal during growth. The gel is thought to work as ferroconcrete supporting the crystals. This appears to be the reason for the greater strength behind crystals grown in gellified gel.
Furthermore, the group experimented with soaking avidin crystals grown in gellified gel in a biotin solution dissolved with high-concentration organic solvents. As a result, the group succeeded in producing crystals of a complex of biotin and avidin without the crystals being damaged by osmotic shock. The group examined the stereoscopic structure of these complex crystals and observed biotin binding with a pit in avidin. (Figure 7)
The significance of this research
This group developed a new technique for growing protein crystals in gellified gel. This technique was found to increase the mechanical stability of protein crystals. This is a world first feat defying common wisdom. Through this technique, it will be possible to analyze structure of crucial proteins involved in diseases, something which has been difficult due to damage to crystals. Furthermore, protein crystals grown by this technique can avoid osmotic shock so it will be easy to produce crystals of complexes of target proteins and lead compounds dissolved in high-concentration organic solvents. This technique will be applied to screening for drug discovery, the process of finding keys for complex keyholes, a process by which drugs are discovered from a large numbers of lead compounds.
Thesis title : “ Growth of Protein Crystals in Hydrogels Prevents Osmotic Shock ” J. Am. Chem. Soc. 134, 5786-5789 (2012)
References
1.“Effect of evaporation on protein crystals grown in semi-solid agarose hydrogel” Jpn. J. Appl. Phys. 50, No.025502 (2011)
2. “Protein Crystallization in Agarose Gel with High Strength: Developing an Automated System for Protein Crystallographic Processes” Jpn. J. Appl. Phys. 48, No.075502 (2009)

Figure 1

Figure 2
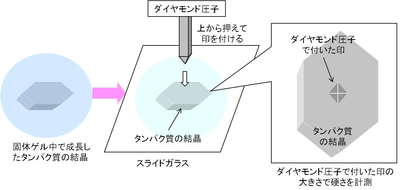
Figure 3
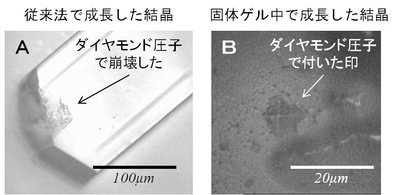
Figure 4

Figure 5
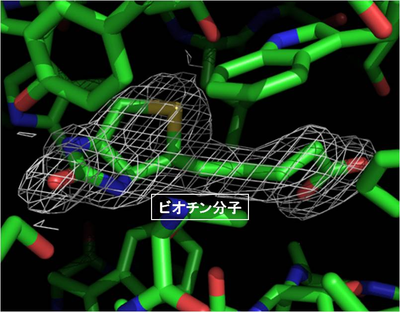
Figure 6
Related link:
