Workings of the bacterial flagellar motor clarified
assembly and anchoring of the stator to the motor as well as ion translocation are linked
A group of researchers from Osaka University and Nagoya University solve the structure enabling the workings of a bacterial flagellar motor.
Project Leaders:
• Graduate School of Science, Osaka University -- IMADA Katsumi , Professor,
• Graduate School of Science, Nagoya University -- KOJIMA Seiji , Associate Professor and HOMMA Michio , Professor
Powered by the electrochemical gradient of its coupling ion as its energy source, the flagellar motor rotates at a high speed with almost 100 percent energy conversion efficiency. It was found that when the stator was incorporated into a motor, part of the folded stator grew and bonded with the cell wall, thereby, activating the motor. The clarification of this basic operating principle is very important in developing promising super high efficient motors and nano-motors.
Bacteria swim in the water by rotating their helical flagellar filament like a screw. This rotation is driven by protein (about 45 nano meters in diameter) at the base of the filament which goes through the cell wall. When ion flows in the stator unit in the motor, torque is generated through the interaction between the stator and the rotor ring. In order to rotate, the rotor needs to be attached in such a way that it can withstand the torque. That's why the stator unit of the motor has a region anchoring to peptidoglycan layer equivalent to a bacterial cell wall. Each stator unit is assembled around the rotor and firmly anchored to the cell wall. When ions start to flow into the stator unit, the motor is activated.
A dozen stators of the flagellar motor are assembled or disassembled from the rotating motor. In the case of the latter, ions do not flow in the disassembled stators. In this way, assembly and anchoring of the stator to the motor as well as ion translocation are linked, but how the stator is assembled into the motor and how ion flow occurs only when it is assembled in the cell wall has been unknown.
This joint group paid attention to the structure of a fragment of the sodium-driven stator protein PomB (PomBc), the region anchoring the stator unit of the sodium-driven flagellar motor of Vibrio. This group analyzed its molecular conformation and found that part of the stator extended on the N-terminal side of PomBc and bonded with the cell wall and that this conformation change was reversible -- leading to expansion or contraction. As cross-linking does not affect the stator assembly and ion translocation, the conformational change of bonding to the cell wall occurred after the stator assembly to motor and ion translocation, that is, the order of activation of flagellar motor was clarified.
Furthermore, there is a close relationship between the pathogenicity and the motility of bacteria. In vibrio cholera and other bacteria, the motility serves as a key to developing disease. Thus, understanding and control of motility is a major challenge in bacteriology. This group's achievement is also important in terms of the control of pathogen motility.
Abstract
The bacterial flagellum is a motility organelle that consists of a rotary motor and a helical propeller. The flagella usually work individually or by forming a loose bundle to produce thrust. However, the flagellar apparatus of marine bacterium MO-1 is a tight bundle of seven flagellar filaments enveloped in a sheath, and it has been a mystery as to how the flagella rotate smoothly in coordination. Here we have used electron cryotomography to visualize the 3D architecture of the sheathed flagella. The seven filaments are enveloped with 24 fibrils in the sheath, and their basal bodies are arranged in an intertwined hexagonal array similar to the thick and thin filaments of vertebrate skeletal muscles. This complex and exquisite architecture strongly suggests that the fibrils counterrotate between flagella in direct contact to minimize the friction of high-speed rotation of individual flagella in the tight bundle within the sheath to enable MO-1 cells to swim at about 300 µ m/s.

Figure 1
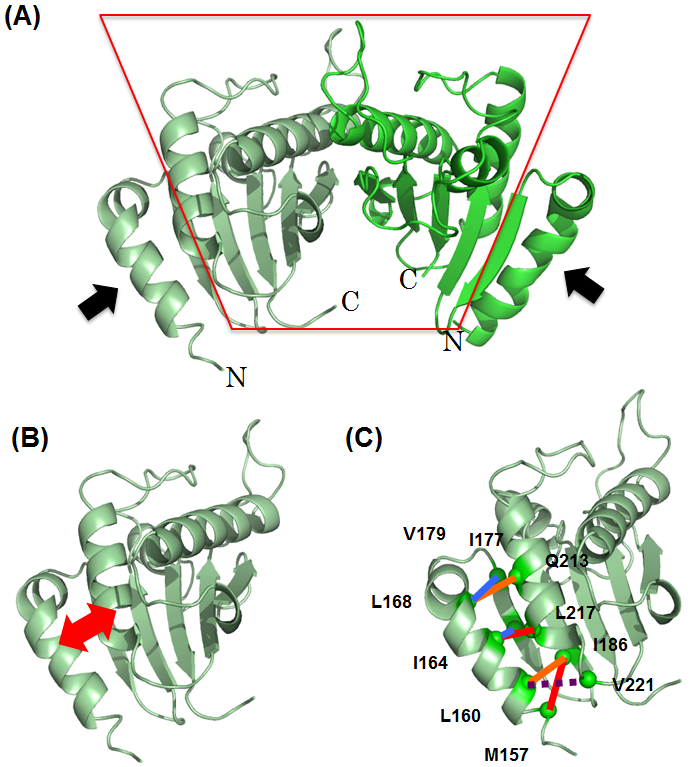
Figure 2

Figure 3

Figure 4
To learn more about this research, please view the full research report entitled " Conformational change in the periplamic region of the flagellar stator coupled with the assembly around the rotor " at this page of the Proceedings of the National Academy of Sciences (PNAS) website.
Related link :
