Structure of protein secretion device of germs clarified at the atomic level
Origin of ATPases are hair and venomous needle?
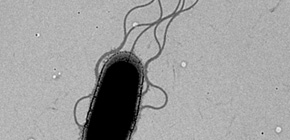
Pathogenic bacteria directly send etiological proteins to cells in humans, animals, and plants through an organ called the "type III secretion system" and pass the infection on to them. Since the type III secretion system is a kind of "stinger" for the bacterium and necessary for infection, research on it is being conducted around the world, but its mechanism is not well known.
A flagellum consists of tens of thousands of proteins. After proteins are produced in the fungus body, they are transported to outer membranes through the flagellar type III secretion system at the base of a flagellum, and are then sent to the tip of the growing flagellum.
Professor IMADA Katsumi (Graduate School of Science, Osaka University) and Associate Professor MINAMINO Tohru (Graduate School of Frontier Biosciences, Osaka University) clarified the complex structure of ATP hydrolytic enzymes and stators which work in the bacterial protein secretion system (type III secretion system) at the atomic level.
From the structure clarified in this study, it was demonstrated that the type III secretion system corresponded to the ancestral type of ATPase and V-type ATPases, which are universally observed in organisms.
If the mechanism of type III secretion is clarified and drugs for preventing transport of pathogenic proteins are developed, it will become possible to take away only pathogenicity without killing bacteria and develop drugs for infectious diseases with less adverse effects and drug-resistant strains. Furthermore, it will be also helpful in studying V-type ATPase, a target of treating cancer and osteoporosis.
Abstract
FliI and FliJ form the FliI 6 FliJ ATPase complex of the bacterial flagellar export apparatus, a member of the type III secretion system. The FliI 6 FliJ complex is structurally similar to the α 3 β 3 γ complex of F 1 -ATPase. The FliH homodimer binds to FliI to connect the ATPase complex to the flagellar base, but the details are unknown. Here we report the structure of the homodimer of a C-terminal fragment of FliH (FliH C2 ) in complex with FliI. FliH C2 shows an unusually asymmetric homodimeric structure that markedly resembles the peripheral stalk of the A/V-type ATPases. The FliH C2 –FliI hexamer model reveals that the C-terminal domains of the FliI ATPase face the cell membrane in a way similar to the F/A/V-type ATPases. We discuss the mechanism of flagellar ATPase complex formation and a common origin shared by the type III secretion system and the F/A/V-type ATPases.

Figure 1
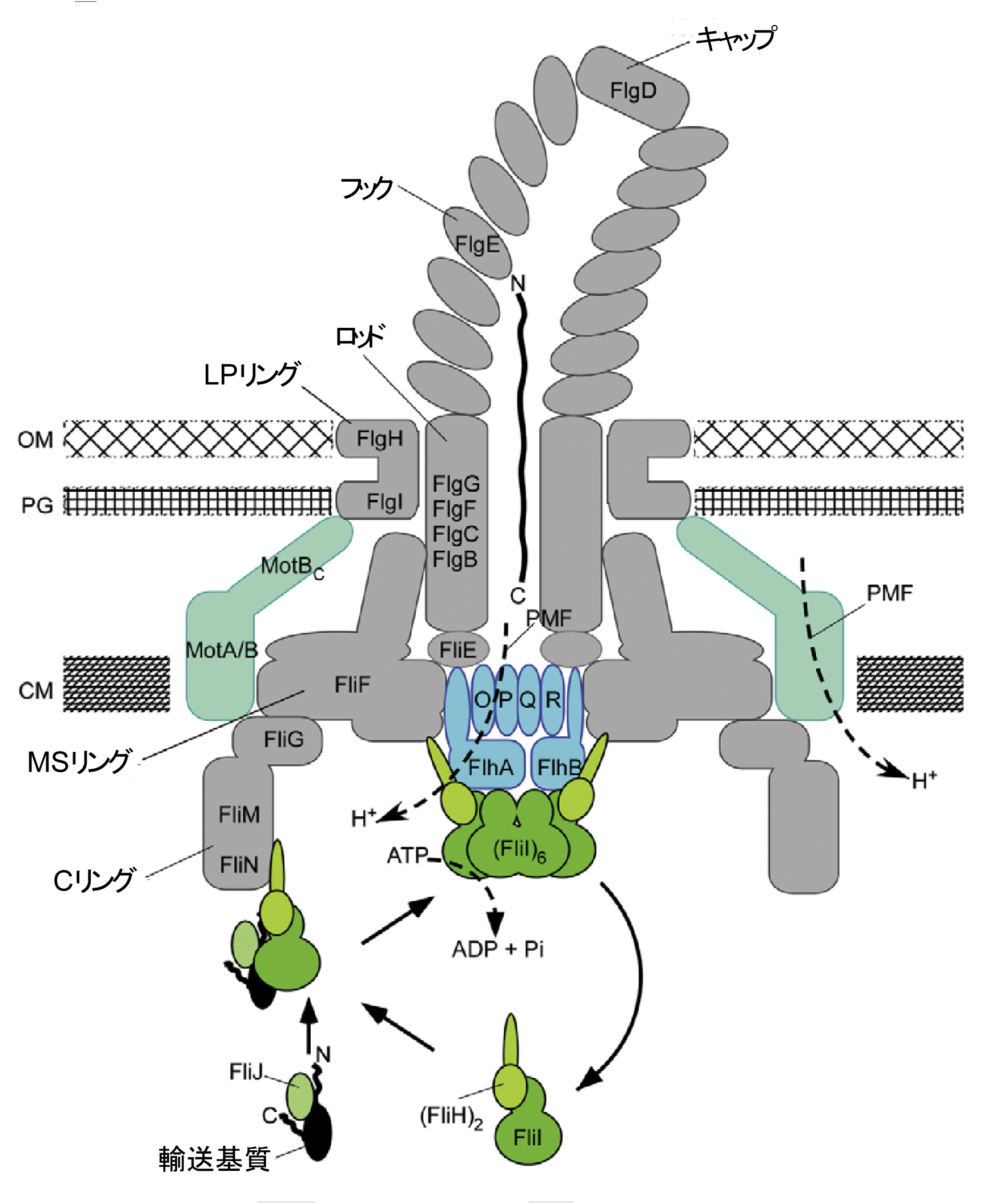
Figure 2
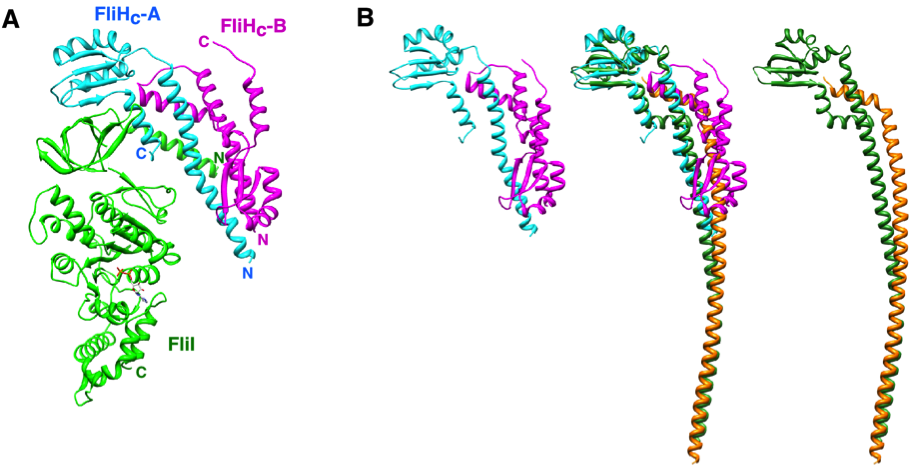
Figure 3
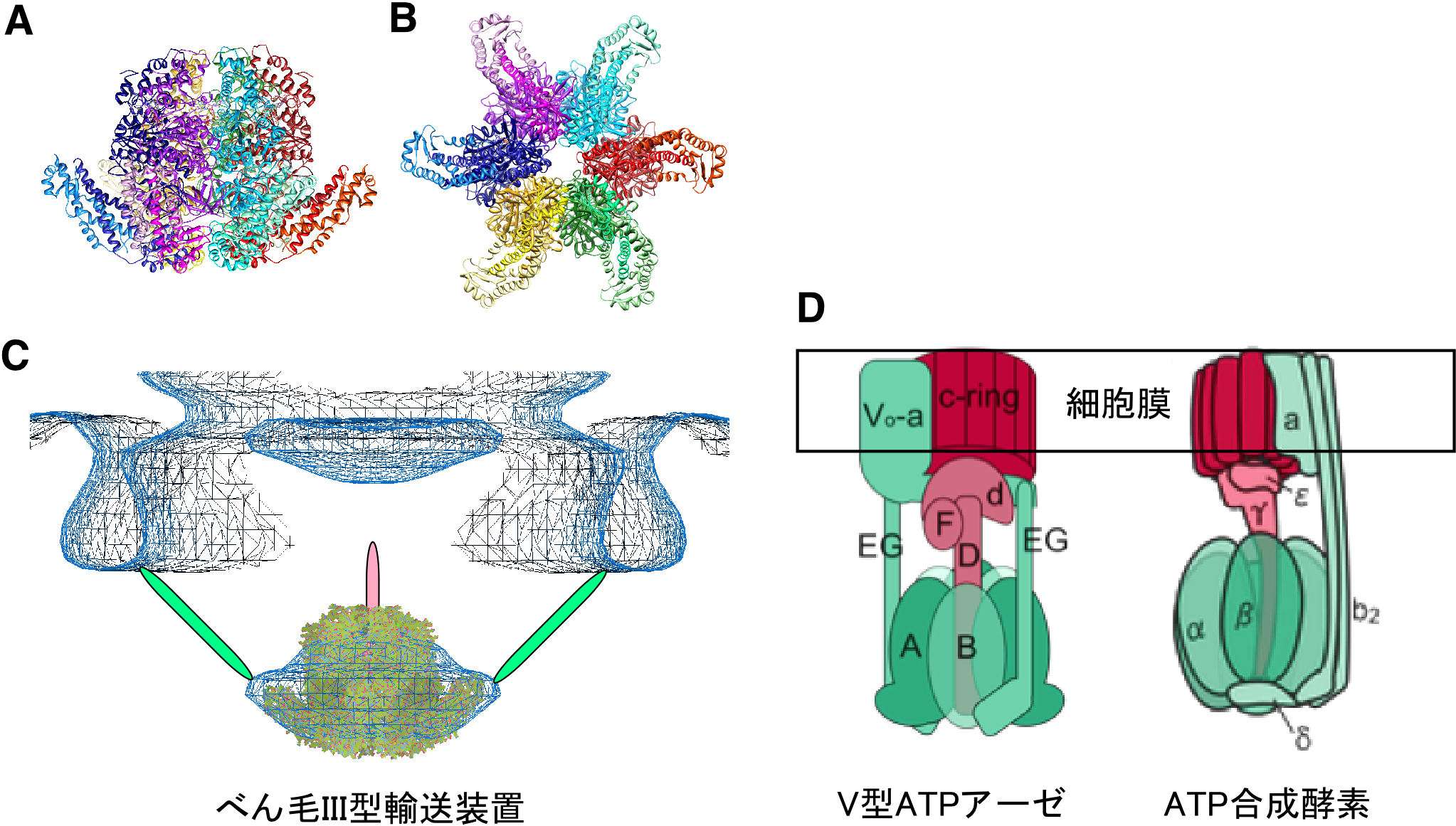
Figure 4
To learn more about this research, please view the full research report entitled " Insight into the flagellar type III export revealed by the complexstructure of the type III ATPase and its regulator " at this page of the Proceedings of the National Academy of Sciences website.
Related links
