How vibrio cholera is attracted by bile elucidated
Toward the prevention of cholera, an infectious disease that threatens the world
A group of researchers from Osaka University, Hosei University and Nagoya University have revealed the molecular mechanism that Vibrio cholerae , the etiological agent of cholera, is attracted by bile.
Background
Cholera, an acute diarrheal disease caused by the infection of the Gram-negative bacterium Vibrio cholerae , remains a global threat to public health. V. cholerae does not produce toxins in nutrient-poor aquatic environments. However, in a nutrient-rich environment, such as the lumen of the human small intestine, it begins to form colonies and expresses pathogenic proteins that cause the serious diarrheal disease. Thus, sensing of environmental chemicals is crucial for pathogenicity of V. cholerae.
V. cholerae is attracted by bile, suggesting that the bacterium senses bile as a chemotactic signal to migrate toward a certain part of intestine. In addition, V. cholerae shows various responses to bile, including synthesis of virulence factors, biofilm formation. Therefore bile-sensing is one of the key properties for its pathogenicity, but it remains unclear how the bacteria sense the bile.
Results
We found that V. cholerae is actually attracted by taurine (2-aminoethylsulfonate), a bile component, and that taurine is recognized by a chemotaxis receptor protein, Mlp37. Mlp37 was originally been identified as a chemoreceptor for various proteinogenic amino acids. The structural study of Mlp37 sensor domain in complex with taurine and serine revealed that the ligands bind to the same pocket and that taurine is recognized essentially in the same way as serine. Interestingly, the sensor domain of the ligand complex had a small opening, which would accommodate a larger side chain group, accounting for the broad ligand specificity of Mlp37. This finding has led us to visualize ligand binding to Mlp37 with a fluorescent derivative of serine whose side chain is decorated by a large fluorescent group. We have successfully visualized the ligand binding to the bacterial chemoreceptor as fluorescent spots. This is the first example of the direct detection of the ligand binding to the bacteria chemoreseptor in vivo .
Impact of research
WHO estimates there are several million cholera cases and more than 100,000 deaths due to cholera every year. Since the discovery of V. cholerae in the 19 th century, mechanisms underlying virulence have extensively been studied, but behaviors of the bacteria in various environments still remain less understood. The finding of taurine taxis shed new light on the survival of V. cholerae in the host intestine as well as its pathogenicity. Inhibition of taurine taxis might lead to prevention of infection and pathogenesis of V. cholerae . The structural basis of taurine recognition by the chemoreceptor Mlp37 provides a great contribution to the development of such new drugs. Moreover, our fluorescent labeling technique provides a powerful cell biological tool to study bacterial chemotactic behavior, which is essential for bacterial survival and infection.
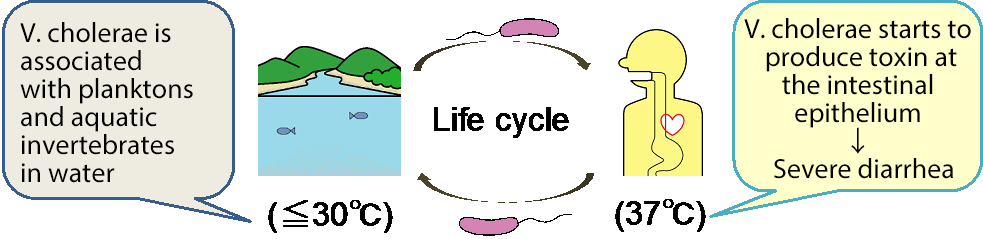
Figure 1. The life cycle of Vibrio cholerae and schematic diagram of V. cholerae attracted by bile in the human body.
V. cholerae does not produce toxins in nutrient-poor aquatic environments, such as rivers and brackish waters. However, in nutrient-rich environments, such as the lumen of the human small intestine, it begins to form colonies and expresses pathogenic proteins that cause the serious diarrheal disease. This study has reveled that V. cholerae is attracted by taurine, a major constituent of bile.
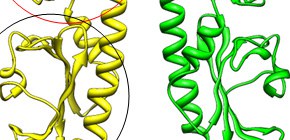
Figure 2. Molecular sturcture of Mlp37
(A) Ribbon representation of Mlp37. Mlp37 forms a dimer (yellow and green). The two domains (the red circle and the black circle) are similar in structure, but only the upper domain binds tauline.
(B) Close up view of the upper domain bound to serine. Mlp37 is colored in pink. The serine is indicated by ball model.
(C) Close up view of the upper domain bound to taurine. Taurine binds to the pocket in the same way as serine. Mlp37 is colored in yellow. The taurine is indicated by ball model.
(D) The structure of Mlp37 bound to serine. The serine is shown by gray balls. The entrance of the binding pocket is slightly open (shown by a black arrow).
The structure of Mlp37 bound to taurine. The taurine is shown by gray and red balls. The entrance of the binding pocket is slightly open (shown by a black arrow).

Figure 3. Visualization of the serine binding to Mlp37 in V. cholerae.
V. cholerae that produces Mlp37 labeled with RFP (red fluorescent protein) binds serine labeled with a green fluorescent dye: left, Mlp37 (red); middle, serine (green); right, merged image. The red fluorescent spots are nicely superimposed to the green ones, indicating Mlp37 binds the fluorescent serine.
To learn more about this research, please view the full research report entitled " Identification of a Vibrio cholera chemoreceptor that senses taurine and amino acids as attractants " at this page of the Scientific Reports website.
Related link
