Ruthenium catalysts with tens of times higher catalytic performance than rhodium catalysts developed
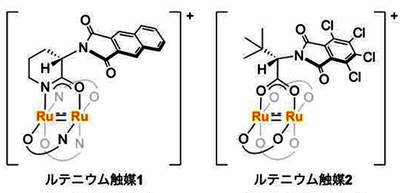
A group of researchers from Hokkaido University, Osaka University, Musashino University, and Rigaku Corporation developed two chiral paddle-wheel diruthenium complexes bearing amidate or carboxylate ligands.
Chirality is the quality of an object to be non-superimposable on its mirror image. Two chiral stereoisomers that are mirror images of each other are called enantiomers. Enantioselectivity is the preferential interaction with the chiral selector of one enantiomer over the other, so the enantioselective synthesis is asymmetric synthesis.
Enantiomers are used as ingredients for medicine. One enantiomer may have a desired beneficial effect, whereas the other may cause dangerous side effects, so a technique to make 2 enantiomers as 2 separate drugs with different pharmaceutical effects is important.
Asymmetric synthesis via chiral catalysts is used for producing isomers; however, a large quantity of expensive catalysts is necessary, so the choice of chiral catalysts widely used was limited.
Chiral paddle-wheel diruthenium complexes are prominent catalysts used for various asymmetric transformations by tuning the structure of the bridging chiral ligands. Thus, chiral paddle-wheel complexes have been used extensively across a broad range of synthetic transformations, but the transition metals incorporated into the reactive site for chiral paddle-wheel complexes have been limited to rare and expensive rhodium.
Of the chiral diruthenium complexes developed by this group, [Ru2((S)-BPTPI)4] exhibited exceptionally high reactivity and enantioselectivity in catalytic asymmetric synthesis of C-C bond-forming reactions. The observed turnover number (TON) was nearly 40 times higher than the previous highest value recorded for hetero-Diels-Alder (HDA) reactions.
Since [Ru2((S)-BPTPI)4] demonstrated remarkable catalytic performance and robustness in asymmetric C–C and C–N bond-forming reactions, in which good results are not obtained with rhodium catalysts, this complex can be used for various reactions. With only 0.00005–0.01 mol% catalyst loading, [Ru2((S)-BPTPI)4] exhibited remarkable reactivity, achieving a TON of up to 1,880,000 with high enantioselectivity.
On the other hand, another diruthenium catalyst developed by this group, [Ru2((S)-TCPTTL)4] exhibited good reactivity and high enantioselectivity in C–H amination and cyclopropanation reactions under oxidizing conditions using hypervalent iodine(III) and in the asymmetric cyclopropanation reactions using iodonium ylide.
Since the diruthenium catalysts have higher oxidation states (Ru(II)–Ru(III)) than the dirhodium catalysts (Rh(II)–Rh(II)), the researchers speculated that the diruthenium catalysts could exhibit much higher Lewis acidity especially in their cationic form. High oxidation tolerance of ruthenium catalysts, namely catalytic stability, is a key to higher performance of ruthenium catalysts than rhodium.
The paddle-wheel diruthenium scaffold is an attractive platform that can facilitate the further development of chiral diruthenium catalysts for a wide range of catalytic asymmetric transformations. The high catalytic activity and stability of paddle-wheel diruthenium complexes will increase the efficiency of chemical synthesis of organic chemicals, leading to the development of medicine.
Figure 1
Figure 2
The article, “Chiral paddle-wheel diruthenium complexes for asymmetric catalysis,” was published in Nature Catalysis at DOI https://www.nature.com/articles/s41929-020-00513-w.
