Functional transmembrane pores useful for applications in biotechnology
A group of researchers from Osaka University, Westlake University (China), and University of Washington (U.S.) has created transmembrane pores with arrangement different from natural ones.
Since malfunction of the cell membrane, which consists of lipids and proteins, causes diseases, researchers study the structure and function of membrane proteins in earnest. Some membrane proteins are known for their sensitivity to ions, so there is a great interest in development of sensors using membrane proteins; however, it is difficult to analyze membrane proteins because they are embedded in a lipid bilayer.
In recent years, de novo proteins have been developed. The “de novo” protein design describes the generation of new proteins with a desired structure by designing amino acid sequences. Computational protein design (CPD) has become a powerful tool to engineer new functional capabilities in proteins using amino acid sequences.
There were numerous examples of successful designs for transmembrane proteins, but transmembrane proteins that have a specific function were not reported and their structures were not clarified.
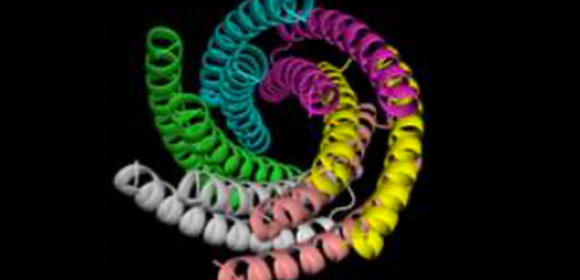
In this study, the researchers designed water-soluble transmembrane pores WSHC6 and then redesigned the lipid-exposed residues in order to convert a water-soluble form into a transmembrane form.
They designed two transmembrane channels: TMHC6 (transmembrane hexameric pore) and TMH4C4 (transmembrane hexameric pore four-helix subunit). TMHC6 has a 12-helix transmembrane channel with an inner diameter of approximately 0.3 nm (3 Å) and TMH4C4 has a 16-helix transmembrane channel with an inner diameter of approximately 1 nm (10 Å).
Using x-ray crystallography and cryogenic electron microscopy (cryo-EM), the researchers clarified that crystal structures of the water-soluble forms of a 12-helical pore and a 16-helical pore closely match the computational design models.
In addition, using a patch-clamp electrophysiology technique, a technique for directly measuring the membrane potential and/or the amount of current passing across the cell membrane, and a liposome-based assay coupled to in vitro protein synthesis, the researchers performed detailed functional analysis of transmembrane pores and found that the in vitro-synthesized TMHC6 and TMH4C4 had similar elution volumes to the bacterially expressed and purified proteins.
Analytical ultracentrifugation (AUC) experiments showed that TMH4C4 sedimented as a tetramer in detergent solution, consistent with the 16-helix design model. They also measured function of the TMHC6 pore and found that the TMHC6 pore clearly had selective transmembrane ion conductance and showed great permeability.
The methods used in this study will be applied to creating new transmembrane pores. The creation of functional transmembrane pores will contribute to the creation of functional transmembrane pores for a wide variety of applications such as biosensors.
Fig. 1
The article, “Computational design of transmembrane pores,” was published in The EMBO Journal at DOI: https://www.nature.com/articles/s41586-020-2646-5.
