Producing H2 from H2O2 using sunlight and photocatalyst -- To use H2O2 as a hydrogen carrier
A group of researchers from the Research Center for Solar Energy Chemistry of Osaka University has developed photocatalyst technology to produce H2 from hydrogen peroxide (H2O2) using phosphoric acid (H3PO4) and metal-free powders (g-C3N4 powders) under sunlight irradiation.
Since hydrogen can be produced from a variety of sources, it contributes to energy risk management. To drastically reduce CO2 emissions, it is necessary to import large amounts of renewable hydrogen energy from overseas. However, H2 gas has a low volumetric energy density and needs to be converted into a storable and transportable liquid energy carrier.
Since H2O2, used as bleach and disinfectant, is also used as fuel for fuel batteries as oxidizing and reductant agents and is storable and transportable, it has drawn attention as an energy carrier. Photocatalytic H2O2 production from O2 and water under visible light irradiation is also being developed.
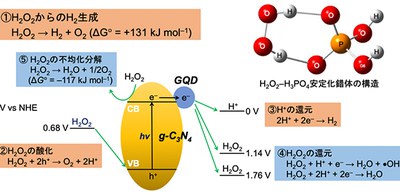
In order to use H2O2 as a hydrogen carrier, on-site generation of H2 from H2O2 solution is necessary. For the development of a sustainable energy society using H2O2 as a hydrogen carrier, the photocatalytic H2O2 splitting into H2 and O2 is a desirable reaction for the generation of H2. However, this reaction is difficult to promote because the photoexcited electrons reduce H2O2 to H2O more preferably than the H+ reduction, suppressing the H2 generation. In addition, H2O2 readily decomposes into water and O2 in the presence of conventional H2 generation photocatalysts on the surfaces of metal-oxide semiconductors. For these reasons, H2 production from H2O2 has not been reported.
This group has conducted research on photocatalysts based on organic semiconductors. Most photocatalysts are semiconductors, which possess a void energy region called a band gap, where no energy levels are available to promote electron and hole recombination by photoactivation. The void energy region extends from the top of the filled valence band to the bottom of the vacant conduction band. Due to the generation of positive holes and excited electrons, photocatalytic reduction and oxidation reactions take place on the surfaces of semiconductors under light irradiation.
When a photon with energy greater than the material's band gap is absorbed by the semiconductor, an electron is excited from the valence band to the conduction band, generating a hole in the valence band.
In this study, visible light irradiation of a graphitic carbon nitride loaded with graphene quantum dots as co-catalysts (GQDs/g-C3N4) in a H2O2 solution containing phosphoric acid (H3PO4) produced H2.
This photocatalyst is photoexcited by absorbing visible light. The photogenerated valence band holes (VB h+) oxidize water (O2 generation) and the conduction band electrons (CB e−) reduce O2 (H2O2 generation). Stabilization of H2O2 by H3PO4 inhibited H2O2 reduction and, hence, promoted H+ reduction, resulting in successful H2 generation. Under visible light exposure, the amount of H2 evolved increases almost linearly with time.
Currently, with the method developed by this group, the H2 selectivity, which is defined as the ratio of the amount of H2 evolved to that of the consumed H2O2, was only ~6%. Thus, it is necessary to increase the H2 selectivity and activity. However, this all-organic photosystem with H3PO4 as a stabilizer will provide a basis of photocatalytic H2O2 splitting and dramatically increase the possible usage of H2O2 as hydrogen/energy carrier.
Figure 1
Figure 2
The article, “Photocatalytic hydrogen peroxide splitting on metal-free powders assisted by phosphoric acid as a stabilizer”, was published in Nature Communications at DOI: https://www.nature.com/articles/s41467-020-17216-2.
