Corrosion-resistant non-noble metal electrodes with high hydrogen generation efficiency produced
Porous graphene improves non-noble metal performances
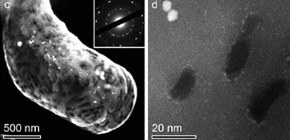
Associate Professor ITO Yoshikazu at the Graduate School of Pure and Applied Sciences, Tsukuba University, together with Assistant Professor OHTO Tatsuhiko at Osaka University, and Professor AJIRI Tadafumi at Tohoku University, fabricated hydrogen evolution electrodes, which were compatible with acid electrolytes and could be applied to electrolysis of water through chemical vapor deposition (CVD) of graphene on a nickel-molybdenum (NiMo) non-noble porous alloy with SiO 2 nanoparticles, a world first.
The holey graphene on the NiMo surface provides non-noble electrodes, which are highly corrosive in acid electrolytes, with two functions:
(1) To minimize corrosion of non-noble metal by avoiding excessive contact between acid electrolyte and the non-noble metal surface,
(2) To give a nano-sized surface area for desirable chemical reaction in which acid electrolyte can make direct contact with the non-noble metal surface.
While existing non-noble metal electrodes corroded in acid electrolyte in less than 10 minutes, the non-noble metal electrodes developed by this group retained initial current density for more than 2 weeks.
Because hydrogen fuel is a zero-emission fuel when burned with oxygen, it has been drawing attention as a clean energy source. Existing hydrogen production methods consume tons of fossil fuel. For hydrogen to become a clean energy source, methods for efficiently generating hydrogen by using recyclable energy should be established.
This group focused on the hydrogen generation system "HHOG" using polymer electrolyte membrane (PEM), which can produce high-purity hydrogen and has high energy conversion rate. In this method, noble metal such as platinum (Pt) is used in electrodes because it is most active and does not corrode in strong acid electrolyte (high durability). However, non-noble carbon metal composite materials have been sought after as an alternative to expensive Pt.
Pt used in electrodes in the clean generation process of hydrogen (electrolysis) costs 3,800 yen per gram, so it’s necessary to reduce the amount of Pt; however, the electrode developed by this group can be synthesized at one hundredth the conventional cost. So, it is expected that electrodes will be produced at low costs through mass production. This electrode production method allows for the continuous production of porous non-noble metal and CVD of graphene in one step, so it is suitable for mass production.
The possibility of applications of non-noble metal, which corrodes in acidic conditions, will increase. In addition to the application to hydrogen generation electrodes, a wide variety of applications, such as solid catalysts, electrodes for fuel cells, supercapacitors, and batteries, are anticipated. This group plans to pursue collaboration with corporations toward the realization of these applications.
Abstract
The development of noble-metal-free hydrogen evolution reaction (HER) materials for electrochemical water splitting is the key to achieving low-cost and efficient electrocatalysis that drives electrochemical hydrogen evolution. However, the electrocatalytic activities of most non-noble metals decrease in acidic electrolytes. Here, we have fabricated non-noble-metal electrodes using a bicontinuous and open porous NiMo alloy covered by nitrogen-doped (N-doped) graphene with nanometer-sized holes. This noble-metal-free HER catalyst exhibits performance almost identical with that of a Pt/C electrode, while its original catalytic activity is preserved even in acidic electrolytes. Density functional theory calculations indicate that the interfacial fringes between the nanoholes and NiMo surface induce charge transfer and promote hydrogen adsorption and desorption. The nanometer-sized holes simultaneously provide minimal surface area for chemical reactions, while delaying NiMo dissolution in excessive amounts of acidic electrolyte. Our method for the fabrication of the NiMo alloy provides a route to a promising class of electrochemical hydrogen-producing electrodes.
Figure 1
Figure 2
Figure 3
Figure 4
To learn more about this research, please view the full research report entitled " Cooperation between holey graphene and NiMo alloy for hydrogen evolution in acidic electrolyte " at this page of ACS Catalysis .
Related link
