Genome does not like to excessively change in male germ cells
A group of researchers at Osaka University reported the function of GTSF1 in male germ cells. The study, which can be read in EMBO Reports , shows that GTSF1 is an essential factor for secondary piRNA biogenesis by regulating piRNA-mediated cleavage of target RNA. The discovery gives important insights on how male germ cells avoid the genome instability commonly seen in other types of cells.
Because a genome codes the entire body, one could naturally assume that mutations should be avoided and that DNA fidelity in the genome is always preserved. The discovery of retrotransposons upset this expectation. These elements are DNA sequences that can copy and paste themselves into another genomic loci. Rather than being undesirable, evolution has selected genomes to include retrotransposons.
Germ cells, which produce sperm and eggs, have a special mechanism to protect the genome from the threat of retrotransposons. While an unstable genome can be potentially advantageous to evolution, it is not when generating offspring. Indeed, germ cells have evolved to produce a distinctive type of RNA, piRNA, that suppresses retrotransposons.
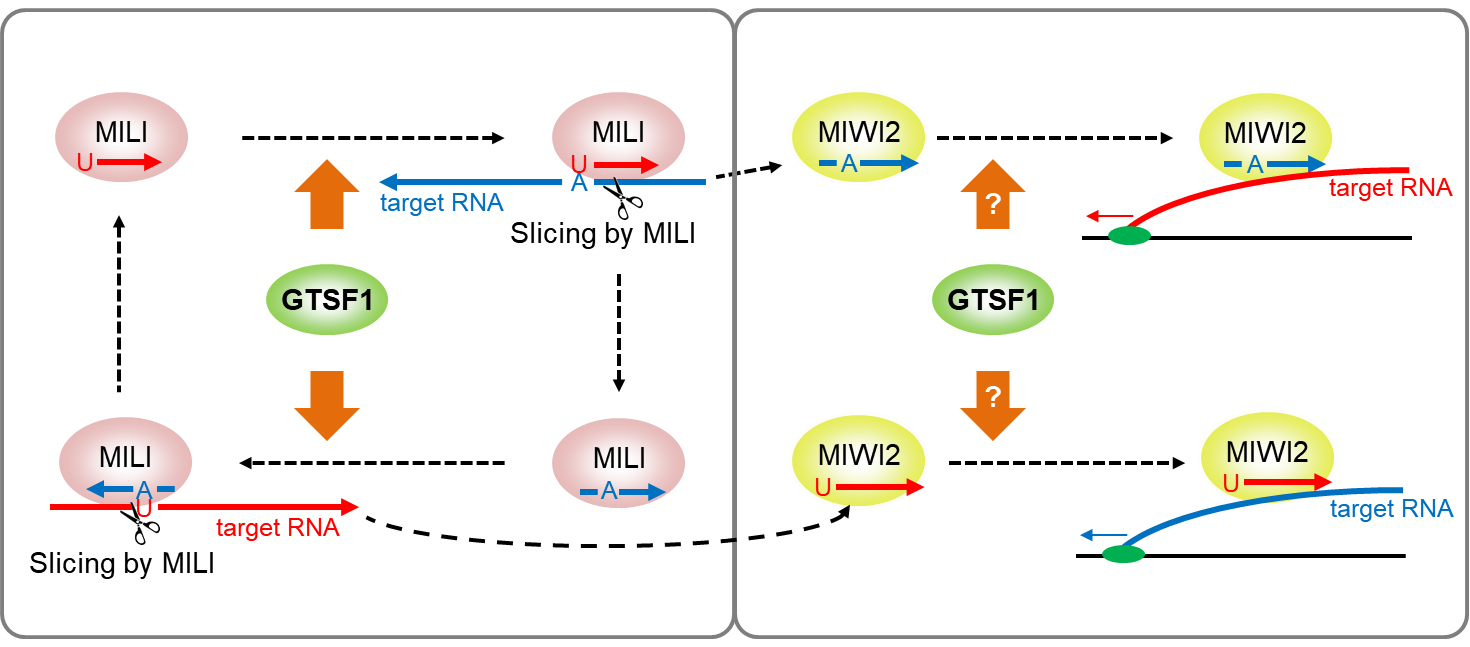
Retrotransposons are suppressed by the primary piRNA biogenesis pathway, secondary piRNA biogenesis pathway (also called the ping-pong cycle), and epigenetic transcriptional silencing in the piRNA pathway. The primary piRNA biogenesis pathway is thought to be independent of guiding based on the sequence of piRNA, whereas the latter two mechanisms are dependent on that.
“In the ping-pong cycle, sense piRNAs process target antisense RNAs to produce antisense piRNAs, and antisense piRNAs process target sense RNAs to produce sense piRNAs having the same sequence as the original sense piRNAs. The ping-pong cycle processes retrotransposon RNAs, whereas the epigenetic transcriptional silencing mechanism targets nascent retrotransposon RNAs to result in suppressive marks in retrotransposon DNA,” explains Osaka University Professor Jun-ichi Miyazaki, an expert in mammalian reproduction and who headed the new study.
MILI and MIWI2 are two core proteins that silence retrotransposons by binding to piRNAs. The study shows that GTSF1, a protein necessary for fertility, binds to both these protein complexes, MILI-piRNA and MIWI2-piRNA, for them to exert their function. Mouse GTSF1 has been studied by the lab, but how it exerts these molecular effects have remained unknown.
“We had identified that mouse GTSF1 is preferentially expressed in male germ cells. We also showed that its loss upregulates the expression of retrotransposons. What we did not know was the mechanism,” says Dr. Takuji Yoshimura, a collaborator of the Miyazaki lab whose research is focused on GTSF1.
The lack of GTSF1 in mouse germ cells nullify the slicing of a known target RNA by MILI-piRNA complex, which is a critical step in the ping-pong cycle. On the other hand, the scientists in the fly piRNA pathway had found that the loss of GTSF1 specifically disrupted the step involving transcriptional silencing in the piRNA pathway. “Mouse GTSF1 may be involved in guiding mechanisms of both ping-pong cycle and transcriptional silencing in the piRNA pathway,” says Dr. Yoshimura.
“The piRNA pathway is a powerful adaptive intracellular immune function for protecting the genome. Elucidating its molecular mechanisms provides a basic understanding of how genome integrity is maintained in organisms including humans,” says Professor Miyazaki.
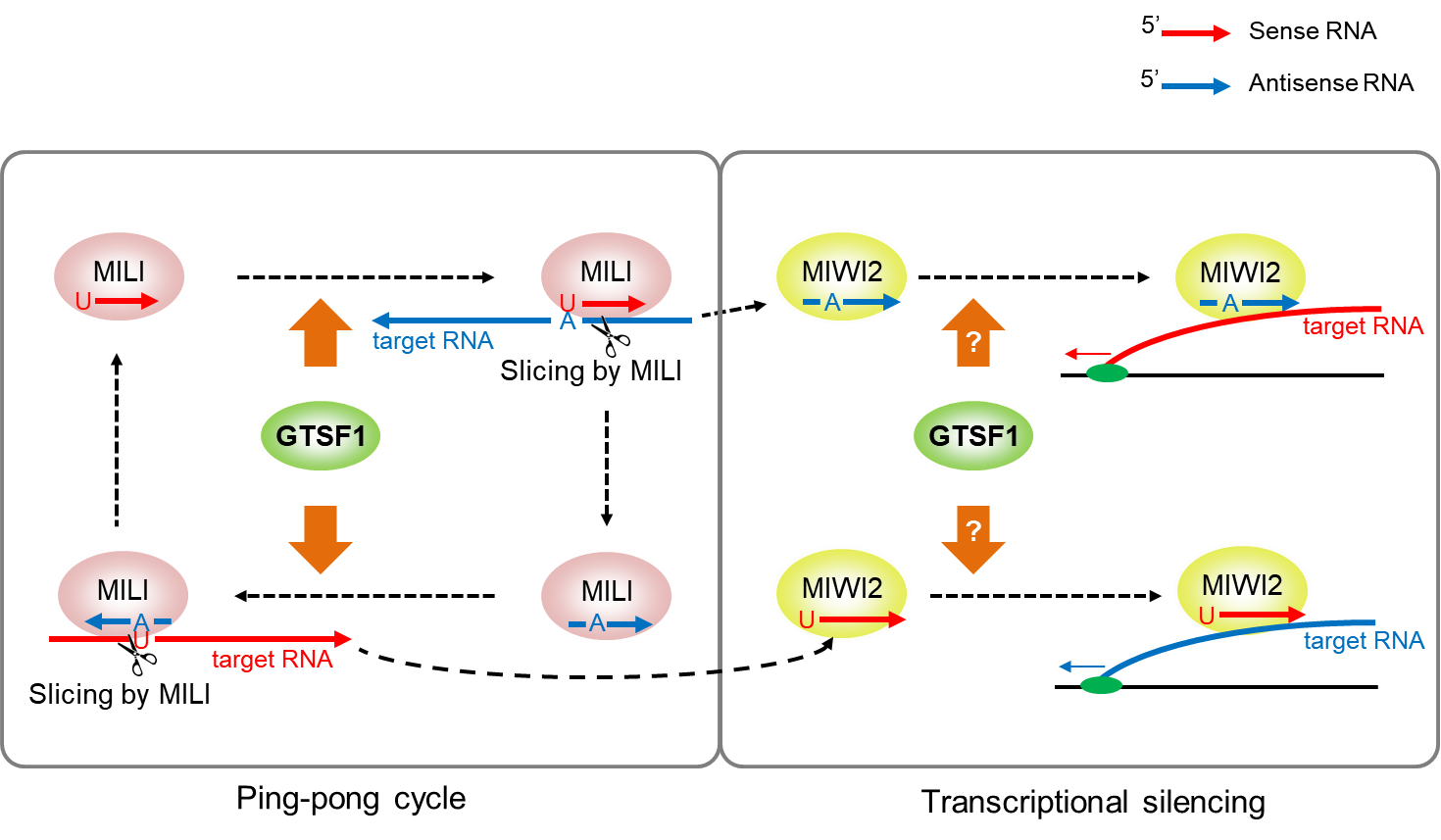
Fig. 1: Mouse GTSF1 is essential for MILI‐directed secondary piRNA biogenesis and associates with the MIWI2 complex probably to transcriptionally silence its targets.
©︎2018 Yoshimura et al. EMBO reports e42054. doi: 10.15252/embr.201642054
To learn more about this research, please view the full research report entitled " Mouse GTSF1 is an essential factor for secondary piRNA biogenesis " at this page of EMBO Reports.
Related links
- Stem Cell Regulation Research, Graduate School of Medicine, Osaka University (link in Japanese)
