Using DNA to predict schizophrenia and autism
Osaka University researchers show in a multi-institute collaboration that a single amino acid substitution in the protein CX3CR1 may act as predictor for schizophrenia and autism
Huntington's disease, cystic fibrosis, and muscular dystrophy are all diseases that can be traced to a single mutation. Diagnosis in asymptomatic patient for these diseases is relatively easy – You have the mutation? Then you are at risk. Complex diseases, on the other hand, do not have a clear mutational footprint. A new multi-institutional study by Japanese researchers shows a potential rare gene mutation that could act as a predictor for two neurodevelopmental disorders, schizophrenia and autism.
"Aberrant synapse formation is important in the pathogenesis of schizophrenia and autism," says Osaka University Professor Toshihide Yamashita, one of the authors of the study. "Microglia contribute to the structure and function of synapse connectivities."
Microglia are the only cells in the brain that express the receptor CX3CR1. Mutations in this receptor are known to affect synapse connectivity and cause abnormal social behavior in mice. They have also been associated with neuroinflammatory diseases such as multiple sclerosis, but no study has shown a role in neurodevelopment disorders.
Working with this hypothesis, the researchers conducted a statistical analysis of the CX3CR1 gene in over 7000 schizophrenia and autism patients and healthy subjects, finding one mutant candidate, a single amino acid switch from alanine to threonine, as a candidate marker for prediction.
“Rare variants alter gene function but occur at low frequency in a population. They are of high interest for the study of complex diseases that have no clear mutational cause,” said Yamashita, who added the alanine threonine substitution was a rare variant.
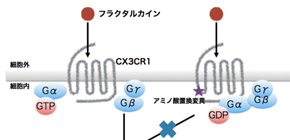
The structure of CX3CR1 includes a domain known as Helix 8, which is important for initiating a signaling cascade. Computer models showed that one amino acid change is enough to compromise the signaling.
"The variant changes the region from hydrophobic to hydrophilic and destabilize Helix 8. We overexpressed the mutation in cells and found Akt signaling was disrupted," explains Yamashita.
According to Yamashita, the findings are the first to connect a genetic variation in microglia with neurodevelopment disorders. Moreover, he hopes that the discovery could become a basis for predictive diagnostics.
"There is no reliable way to diagnose schizophrenia or autism in asymptomatic patients. Deeper understanding of the genetic risk factors will help us develop preventative measures."
Abstract
CX3CR1, a G protein-coupled receptor solely expressed by microglia in the brain, has been repeatedly reported to be associated with neurodevelopmental disorders including schizophrenia (SCZ) and autism spectrum disorders (ASD) in transcriptomic and animal studies but not in genetic studies. To address the impacts of variants in CX3CR1 on neurodevelopmental disorders, we conducted coding exon-targeted resequencing of CX3CR1 in 370 Japanese SCZ and 192 ASD patients using next-generation sequencing technology, followed by a genetic association study in a sample comprising 7054 unrelated individuals (2653 SCZ, 574 ASD and 3827 controls). We then performed in silico three-dimensional (3D) structural modeling and in vivo disruption of Akt phosphorylation to determine the impact of the detected variant on CX3CR1-dependent signal transduction. We detected a statistically significant association between the variant Ala55Thr in CX3CR1 with SCZ and ASD phenotypes (odds ratio = 8.3, P = 0.020). A 3D structural model indicated that Ala55Thr could destabilize the conformation of the CX3CR1 helix 8 and affect its interaction with a heterotrimeric G protein. In vitro functional analysis showed that the CX3CR1-Ala55Thr mutation inhibited cell signaling induced by fractalkine, the ligand for CX3CR1. The combined data suggested that the variant Ala55Thr in CX3CR1 might result in the disruption of CX3CR1 signaling. Our results strengthen the association between microglia-specific genes and neurodevelopmental disorders.
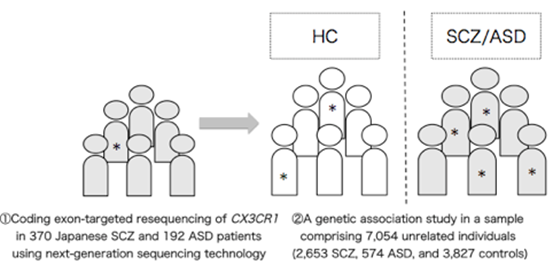
Figure 1. A statistically significant association between the variant Ala55Thr in CX3CR1 with SCZ and ASD phenotypes.
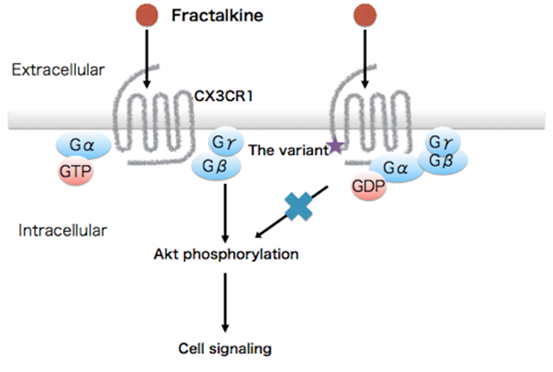
Figure 2
To learn more about this research, please view the full research report entitled " Rare genetic variants in CX3CR1 and their contribution to the increased risk of schizophrenia and autism spectrum disorders " at this page of the Translational Psychiatry website.
Related links
