Nanocatalyst makes heavy work of formic acid
Researchers from Osaka University report an inexpensive and scalable route to hydrogen isotope compounds
Hydrogen occurs in nature as H 2 molecules; however when deuterium isotopes—so called “heavy hydrogen”—are introduced, the result can be deuterium hydride (HD) or deuterium gas (D 2 ). These compounds are useful starting materials in fine chemical production; however, the natural abundance of these gases is low and the techniques used for producing D 2 are expensive and energy intensive. Researchers from Osaka University have now developed a catalyst that promotes selective production of D 2 and HD from the inexpensive starting material formic acid in the presence of D 2 O. Their findings are published in Nature Communications .
Formic acid (FA) has low toxicity, high hydrogen content, and is also low-cost and non flammable, making it an attractive hydrogen storage material. Significant effort has therefore been devoted to optimizing the use of FA as a source of hydrogen. However, previously reported reactions to produce deuterium gases from FA have required the expensive deuterium form of FA as a starting material and high toxicity materials. In addition, the use of homogenous processes, where the catalyst and the reactants are the same phase, has made the recovery of the catalysts challenging and expensive on a large scale.
The researchers report a heterogeneously catalyzed process, in which the catalyst is a different phase to the reactants, using a palladium-based alloy nanocatalyst (PdAg). The catalyst is supported on a silica substrate modified with amine groups that promote the reaction. The amine groups were found to be central to the viability of the reaction and a correlation between the basicity of the amine groups used and the selectivity of the reaction was demonstrated.
“Heterogeneously catalyzed processes are advantageous as they reduce the need for challenging separations,” study lead author Kohsuke Mori explains. “Further advantages of our process are the cost-effective formic acid starting material and the control over the product gas we have demonstrated by tuning the amine groups on the catalyst surface. We also showed that the H/D exchange reaction that leads to the formation of the hydrogen isotope gases involves a quantum tunneling effect.”
Molecules that contain deuterium in place of hydrogen are extremely useful research tools in chemistry and life sciences owing to their distinct properties of deuterium nuclei compared with hydrogen equivalents.
“The demand for deuterated products continues to increase as their applications are developed and become more widespread; therefore, it is important to make the production of precursors more accessible,” study corresponding author Hiromi Yamashita explains. “Japan in particular relies heavily on imported materials, so we hope that our catalyst will lead to viable low-cost systems that will be able to satisfy the increasing global demand.”
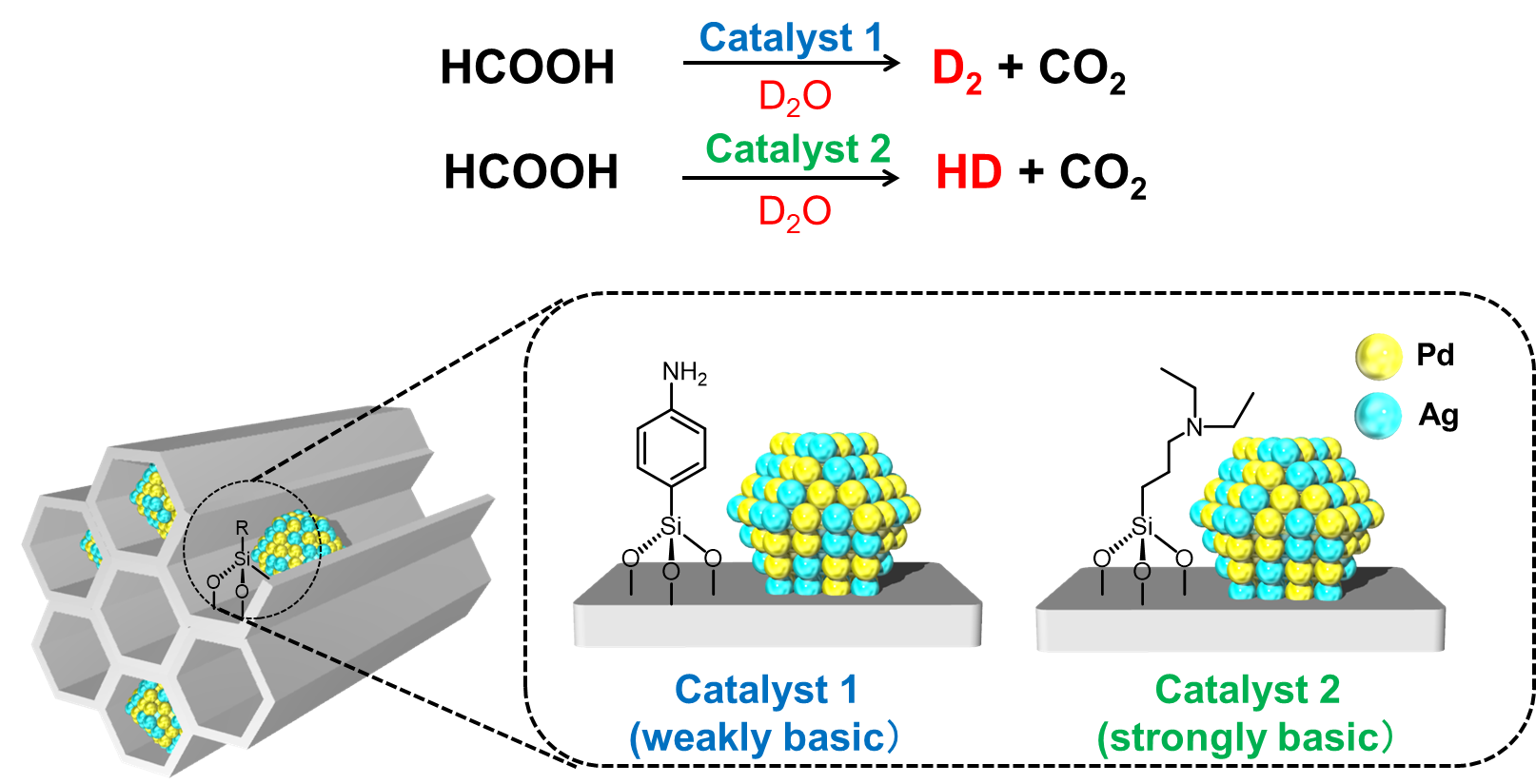
Fig.1. Controlled release of hydrogen isotope compounds from the dehydrogenation of formic acid in D 2 O by the PdAg alloy nanoparticles-supported on amine functionalized silica.
The article, “Controlled release of hydrogen isotope compounds and tunneling effect in the heterogeneously-catalyzed formic acid dehydrogenation” was published in Nature Communications at DOI: https://doi.org/10.1038/s41467-019-12018-7.
Related links
