High-value chemical synthesis in butadiene achieved via deactivated catalysts
Will lead to the development of synthesis methods for various organic materials
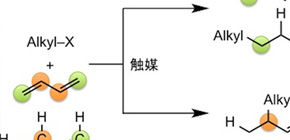
A group of researchers led by KAMBE Nobuaki (Professor) and IWASAKI Takanori (Assistant Professor) at the Graduate School of Engineering, Osaka University, achieved selective alkylation of internal carbon of butadiene by making use of cheap copper catalysts. Contrary to most conventional synthesis reactions that introduce substituent groups on the terminal carbon of butadiene, the reaction achieved by this group is regioselective. With this group's method, it is possible to synthesize high-value terminal olefins by using inexpensive butadiene.
Abstract
Copper complexes generated in situ from CuCl2, alkyl Grignard reagents, and 1,3-dienes play important roles as catalytic active species for the 1,2-hydroalkylation of 1,3-dienes by alkyl fluorides through C F bond cleavage. The alkyl group is introduced to an internal carbon atom of the 1,3-diene regioselectively, thus giving rise to the branched terminal alkene product.
Figure 1
Figure 2
To learn more about this research, please view the full research report entitled " Cu-Catalyzed Regioselective Hydroalkylation of 1,3-Dienes with Alkyl Fluorides and Alkyl Grignard Reagents " at this page of the Angewandte Chemie website.
Related links
