Biological System for Fate Determination of Macrophages Elucidated
Birth of osteoclastic cells dependent on metabolic state
Bone destruction in chronic inflammation such as rheumatoid arthritis (RA) is mainly attributable to the abnormal activation of osteoclasts, one of the macrophage cells related to immune system.
A group of researchers led by NISHIKAWA Keizo (Assistant Professor) and ISHII Masaru (Professor) at the Immunology Frontier Research Center at Osaka University has clarified that differentiation and fate determination of osteoclasts is affected not only by cytokine, but also by the environment where the cell is placed, especially a metabolic state in the cell.
This group has found that:
- 1. Aerobic metabolism of osteoclasts increases the production of S-adenosyl-methionine (SAM) which is important for osteoclast differentiation.
- 2. SAM's metabolic enzyme Dnmt3a, whose expression is induced by osteoclast differentiation, is involved in gene expression regulation via DNA methylation as well as enhanced osteoclast differentiation. In order to regulate DNA methylation, interaction between Dnmt3a and SAM, epigenetic regulation for connecting osteoclast differentiation with intracellular metabolism, is crucial.
These results suggest that inhibition of Dnmt3a-mediated epigenetic regulation is a potentially effective strategy for preventing bone loss.
Abstract
Metabolic reprogramming occurs in response to the cellular environment to mediate differentiation, although the fundamental mechanisms linking metabolic processes to differentiation programs remain to be elucidated. During osteoclast differentiation, a shift toward oxidative metabolic processes occurs3. In this study we identified the de novo DNA methyltransferase Dnmt3a to be a transcription factor that couples these metabolic changes to osteoclast differentiation. Receptor
activator of nuclear factor-κB ligand (RANKL) is an essential cytokine for osteoclastogenesis that induces a metabolic shift toward oxidative metabolic processes, accompanied by an increase in S-adenosyl methionine (SAM) production. We found that SAM-mediated DNA methylation by Dnmt3a regulates osteoclastogenesis via epigenetic repression of the anti-osteoclastogenic gene and that Dnmt3a-deficient osteoclast precursor cells do not undergo osteoclast differentiation efficiently. The importance of Dnmt3a in bone homeostasis was underscored by the observation that mice with an osteoclast-specific deficiency in Dnmt3a exhibit a high bone mass phenotype due to a smaller number of osteoclasts.
Furthermore, inhibition of DNA methylation by theaflavin derivative abrogated bone loss in models of osteoporosis. Thus, this study reveals the role of epigenetic processes in the regulation of cellular metabolism and differentiation, which may provide the molecular basis for a new therapeutic strategy.
Conceptual figure
Figure 1. Effect of the respiratory chain inhibitors antimycin A (100 nM) and oligomycin (1 nM) on Irf8 expression and SAM levels in BMMs stimulated with or without RANKL.
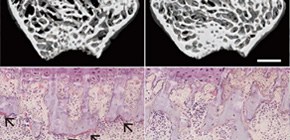
Figure 2. Histological analysis of the proximal tibias of 10-week-old control, Dnmt3aCtsk –/– , and Dnmt3aRank –/– male mice.
Figure 3. The therapeutic effect of the Dnmt3a inhibitor TF-3 on ovariectomy (OVX)-induced bone loss.
To learn more about this research, please view the full research report entitled " DNA methyltransferase 3a regulates osteoclast differentiation by coupling to an S-adenosylmethionine– producing metabolic pathway " at this page of the Nature Medicine website.
Related Links
