Regulatory T cells’ involvement in the progress of colorectal cancers (CRCs) found
Treatment of CRCs by controlling bacteria in intestines is anticipated
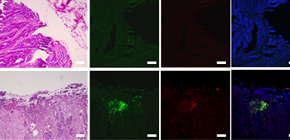
A group of researchers led by Dr. SAITO Takuro, Associate Professor NISHIKAWA Hiroyoshi, and Professor SAKAGUCHI Shimon at the Immunology Frontier Research Center, Osaka University clarified that, in lymphocytes invading deep inside cancer tissue, there are many subpopulations whose expression level of FOXP3 on FOXP3 + cells was low, augmenting antitumor immunity. FOXP3 + cells had been considered regulatory T (Treg) cells.
This group also elucidated that these subpopulations were T cells without suppressive ability and were induced by inflammatory cytokines such as IL-12, which increased by intestinal bacteria attaching to CRCs. (Figure)
CRCs with abundant infiltration of FOXP3 lo T cells showed significantly better prognosis than those with predominantly FOXP3 hi T reg cell infiltration like other cancers, showing that the invasion of Treg cells caused a poor prognosis.
In cancer immunotherapy, Treg cells involved in antitumor immunity have been drawing attention. It is thought that the controlling of immunosuppression by these subpopulations can enhance the efficacy of cancer immunotherapy.
Treg cells are responsible for immunosuppression, and FOXP3 is a master gene, a gene necessary for the function of Treg cells. It is thought that Treg cells are an important factor for cancers to escape from attack from the immune system (mechanism of tumor immune escape) by suppressing various antitumor immune responses.
Therefore, in many cancer tumors, tumor-infiltrating Treg cells has been reported as a factor of a poor prognosis; however, immune response of Treg cells against CRCs had not been clarified.
In recent years, it has been clarified that intestinal flora in the intestine play an important role in regulating immune response and it has been demonstrated that Treg cells were induced by certain types of bacteria. However, how changes in immune response by intestinal bacteria affected CRCs had not been clarified.
The research group led by Professor Sakaguchi had reported that some groups in FOXP3 + cells groups had no suppressive ability. Such subpopulations were a challenge when considering cancer immunotherapy by depleting Treg cells.
By examining tumor-infiltrating lymphocytes in human CRCs in detail, this group elucidated the roles of Treg cells and FOXP3 + cells without suppressive ability and clarified the impact when these cells were induced by intestinal bacteria.
This group’s research has enabled the accurate assessment of FOXP3 + cells in colorectal tumors. Identification of these subpopulations in tumors at the front lines of cancer immunity will become a marker useful for extracting a targeted cancer tumor in developing cancer immunotherapy.
It is known that cancer immunotherapy is effective for only some types of tumors. This group’s research has clarified patients requiring medical attention and the possibility of cancer immunotherapy targeting Treg cells was suggested.
In addition, the possibility that intestinal bacteria can increase antitumor immunity via inflammation in tumors was demonstrated. It is expected that controlling intestinal bacteria will be useful for treating CRCs as well.
Abstract
CD4 + T cells that express the forkhead box P3 (FOXP3) transcription factor function as regulatory T (Treg) cells and hinder effective immune responses against cancer cells. Abundant Treg cell infiltration into tumors is associated with poor clinical outcomes in various types of cancers. However, the role of Treg cells is controversial in colorectal cancers (CRCs), in which FOXP3 + T cell infiltration indicated better prognosis in some studies. Here we show that CRCs, which are commonly infiltrated by suppression-competent FOXP3 hi Treg cells, can be classified into two types by the degree of additional infiltration of FOXP3 lo nonsuppressive T cells. The latter, which are distinguished from FOXP3 + Treg cells by non-expression of the naive T cell marker CD45RA and instability of FOXP3, secreted inflammatory cytokines. Indeed, CRCs with abundant infiltration of FOXP3 lo T cells showed significantly better prognosis than those with predominantly FOXP3 hi Treg cell infiltration. Development of such inflammatory FOXP3 lo non-Treg cells may depend on secretion of interleukin (IL)-12 and transforming growth factor (TGF)-b by tissues and their presence was correlated with tumor invasion by intestinal bacteria, especially Fusobacterium nucleatum. Thus, functionally distinct subpopulations of tumor-infiltrating FOXP3 + T cells contribute in opposing ways to determining CRC prognosis. Depletion of FOXP3 hi Treg cells from tumor tissues, which would augment antitumor immunity, could thus be used as an effective treatment strategy for CRCs and other cancers, whereas strategies that locally increase the population of FOXP3 lo non-Treg cells could be used to suppress or prevent tumor formation.
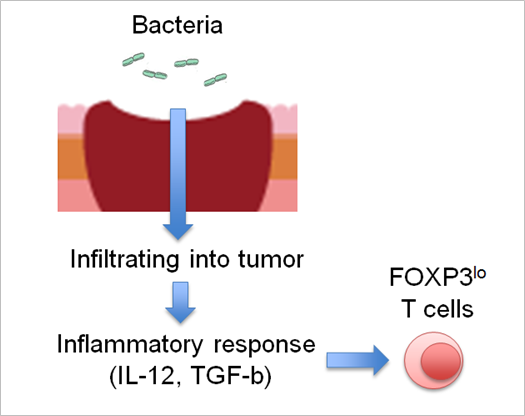
Figure: Bacteria infiltration into colorectal tumors leads to inflammatory response in tumor, which induces FOPX3 lo T cells, then enhancing anti-tumor immunity.
To learn more about this research, please view the full research report entitled " Two FOXP3+CD4+ T-cell subpopulations distinctly control the prognosis of colorectal cancers " at this page of the Nature website.
Related links
