Recovering Lost Skeletal Muscle Function by Using Light Pulses
May lead to possible development of new therapeutic method for complementing or recovering lost skeletal muscle function
A group of researchers developed photosensitive muscle cells by integrating altered light-responsive ion channels (channelrhodopsin) into myoblasts.
This group also succeeded in maturing the cells to skeletal muscle cells having contractile ability by irradiating light on the cells. This technology enables direct optical manipulation of cell differentiation and is expected to become an alternative to conventional electric or drug stimulation.
This technology may lead to the possible development of a new therapeutic method for complementing or recovering lost skeletal muscle function in serious patients with amyotrophic lateral sclerosis (ALS) with extreme muscle weakness.
• Graduate School of Engineering, Osaka University -- ASANO Toshifumi , Assistant Professor (currently Assistant Professor, Tokyo Medical and Dental University)
• The Center for Advanced Medical Engineering and Informatics, Osaka University -- MORISHIMA Keisuke , Professor
• School of Life Science, Tohoku University -- YAO Hiromu , Professor, ISHIZUKA Toru , Lecturer
Abstract
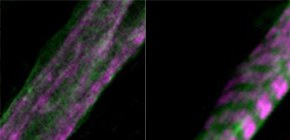
Myoblasts can be differentiated into multinucleated myotubes, which provide a well-established and reproducible muscle cell model for skeletal myogenesis in vitro. However, under conventional differentiation conditions, each myotube rarely exhibits robust contraction as well as sarcomere arrangement. Here, we applied trains of optical stimulation (OS) to C2C12 myotubes, which were genetically engineered to express a channelrhodopsin variant, channelrhodopsin-green receiver (ChRGR), to investigate whether membrane depolarization facilitates the maturation of myotubes. We found that light pulses induced membrane depolarization and evoked action potentials in ChRGR-expressing myotubes. Regular alignments of sarcomeric proteins were patterned periodically after OS training. In contrast, untrained control myotubes rarely exhibited the striated patterns. OS-trained and untrained myotubes also differed in terms of their resting potential. OS training significantly increased the number of contractile myotubes. Treatment with nifedipine during OS training significantly decreased the fraction of contractile myotubes, whereas tetrodotoxin was less effective. These results suggest that oscillations of membrane potential and intracellular Ca 2+ accompanied by OS promoted sarcomere assembly and the development of contractility during the myogenic process. These results also suggest that optogenetic techniques could be used to manipulate the activity-dependent process during myogenic development.
To learn more about this research, please view the full research report entitled "Optogenetic induction of contractile ability in immature C2C12 myotubes" at this page of the Scientific Reports website.
Related links
