Development of promising method of femtosecond crystallography
Makes possible radiation damage-free crystal structure analysis
A group of researchers at Riken , the University of Hyogo , the Japan Synchrotron Radiation Research Institute , Osaka University , and Okayama University succeeded in developing a method of femtosecond X-ray Laser crystallography using X-ray Free Electron Laser (XFEL) at the SPring-8 angstrom compact free-electron laser (SACLA) facility. 2014 marks the 101st anniversary of the first successful X-ray crystallography and is designated as "International Year of Crystallography" by UNESCO and the International Union of Crystallography. This group's technology achieved on this special year can be said to be a milestone marking the beginning of the century of Laser X-ray crystal structure analysis.
If femtosecond X-ray Laser crystallography using laser X-ray at at SACLA is put into practice, radiation damage associated with conventional X-ray crystal structure analysis can be avoided. X-ray crystal structure analysis is an excellent method for determining 3-dimensional atomic structures of substances in a wide area; however, there has been the problem that due to radiation damage from the exposure to X-rays, 3-dimensional atomic structures of substances were occasionally found to be different from the original structure.
This joint group created a device capable of taking X-ray analysis photos by using femtosecond pulses of an XFEL at SACLA and succeeded in developing a method of femtosecond X-ray Laser crystallography. This group also succeeded in clarifying accurate 3D atomic structures of a large membrane protein, cytochrome oxidative enzyme, a key player in oxygen respiration, which had been impossible in conventional X-ray crystal structure analysis due to radiation damage. Thanks to this group's technology, it will become possible to apply fine structure of proteins to manufacturing of high functional catalysts such as artificial photosynthesis catalysts. Moreover, femtosecond X-ray Laser crystallography will become a basic technology for achieving highly accurate high-speed structural analysis for depicting individual moments of protein activity.
Abstract
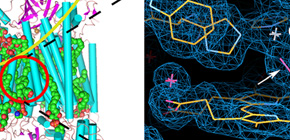
We report a method of femtosecond crystallography for solving radiation damage–free crystal structures of large proteins at sub-angstrom spatial resolution, using a large single crystal and the femtosecond pulses of an X-ray free-electron laser (XFEL). We demonstrated the performance of the method by determining a 1.9-Å radiation damage–free structure of bovine cytochrome c oxidase, a large (420-kDa), highly radiation-sensitive membrane protein.
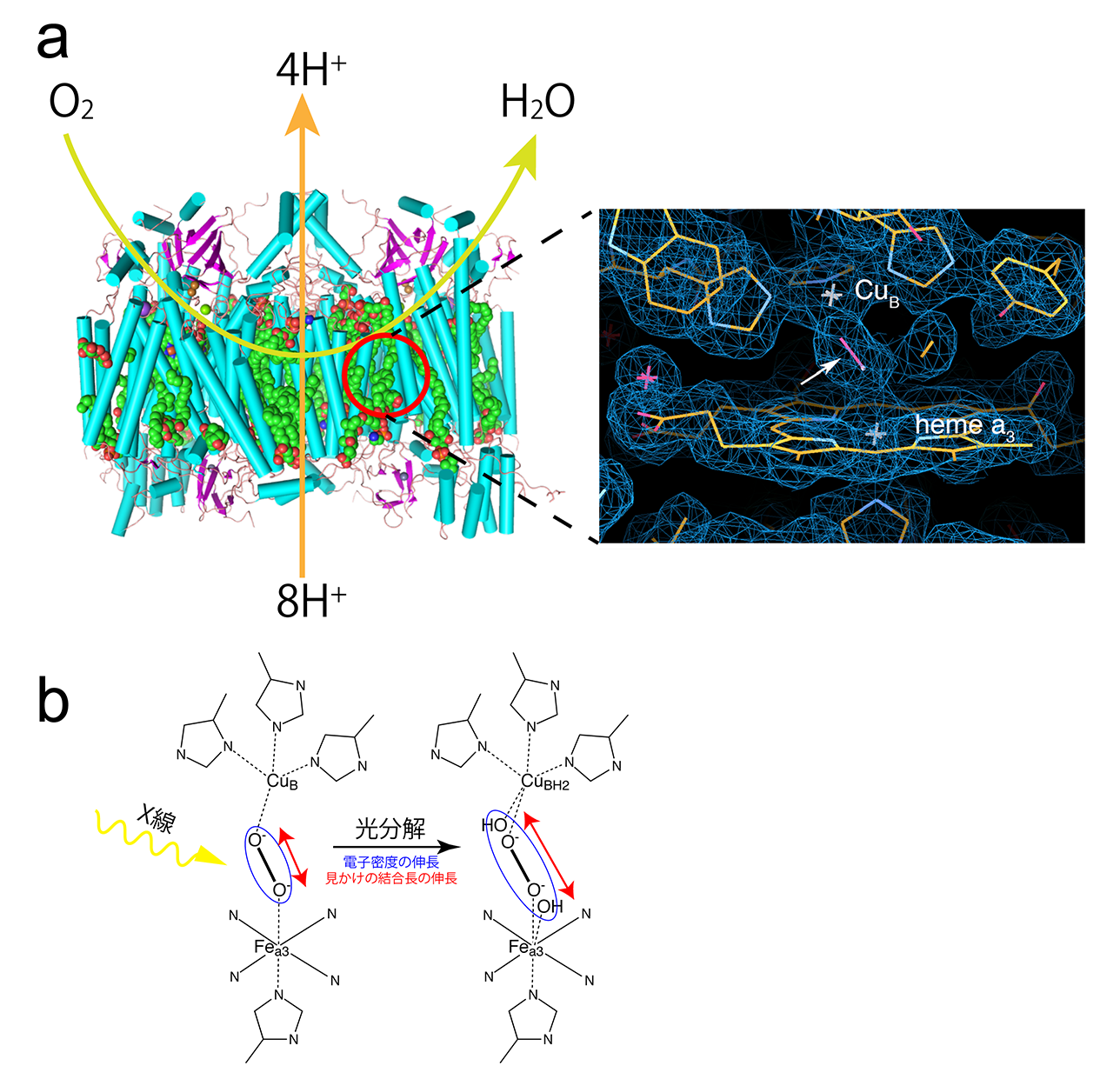
Figure 1
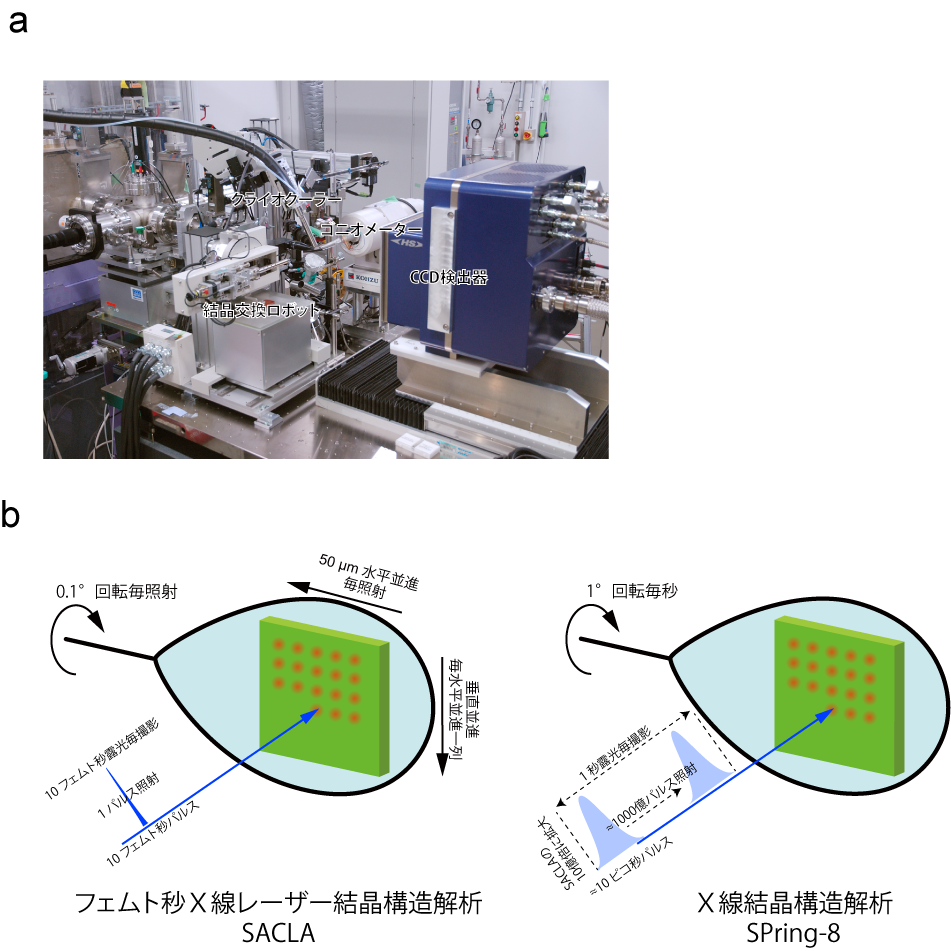
Figure 2
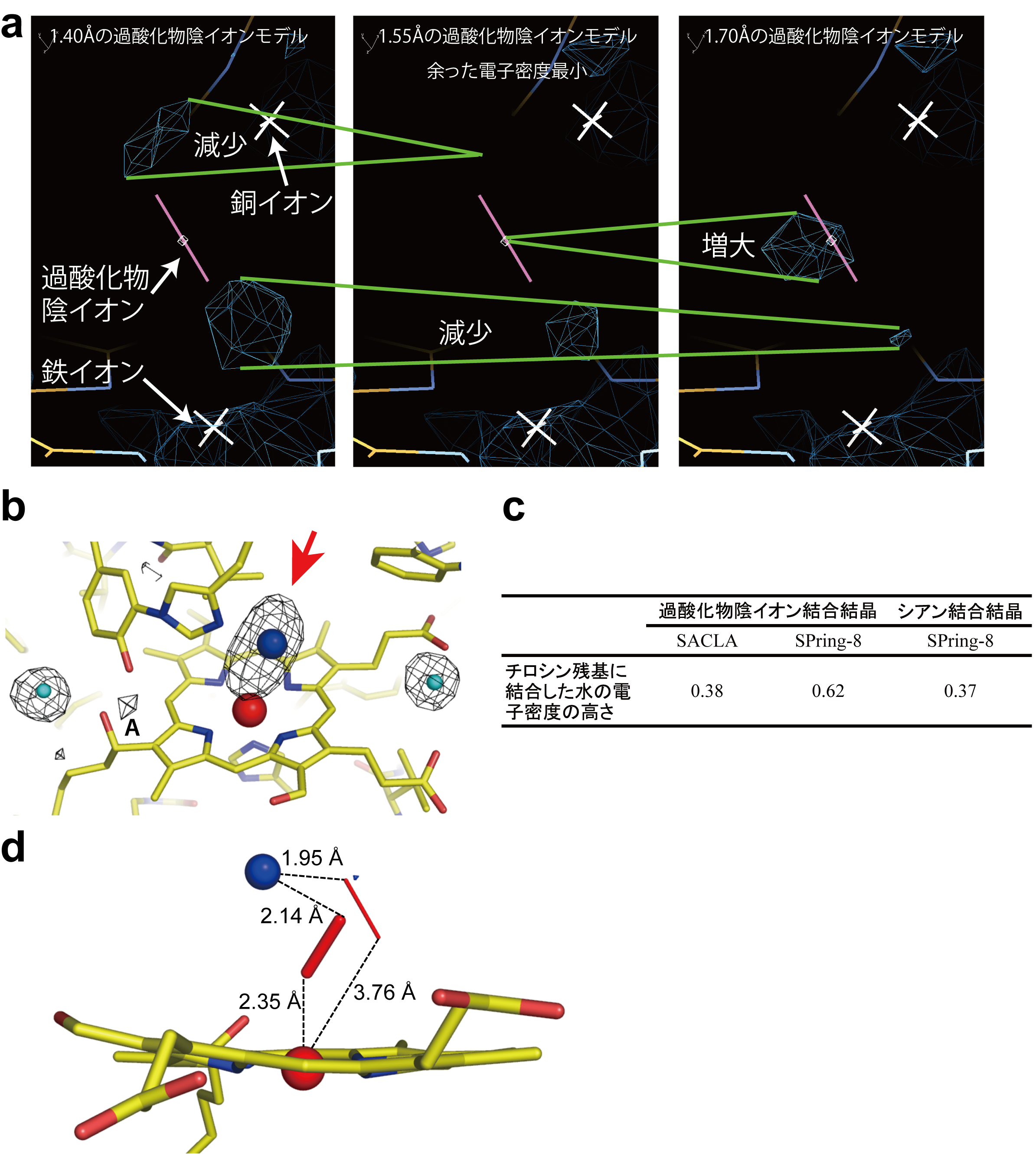
Figure 3
To learn more about this research, please read the full research report entitled " Determination of damage-free crystal structure of an X-ray–sensitive protein using an XFEL " at this page of the Nature Methods website.
Related link :
