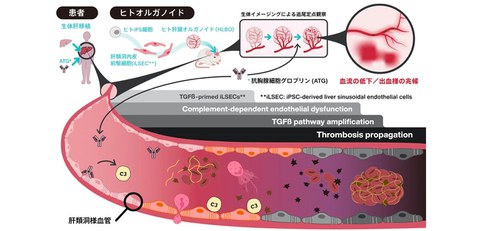
Elucidated mechanism of vascular impairment caused by immunosuppressive agent by using human iPSC-derived vascularized liver organoids
World's first visualization of hepatic microvasculopathy caused by drugs used in transplant medicine
- Established a unique intravital imaging system that enables observation of the dynamics of human blood vessels by transplanting liver organoids with sinusoids created from human iPSCs into immunodeficient mice, while the mice are alive.
- For the first time in the world, researchers elucidated the fact that antithymocyte globulin (ATG), an immunosuppressive agent widely used in transplant medicine, causes microangiopathy in the liver in two stages.
- It was identified that the first stage is rapid thrombus formation due to excessive activation of the complement system (part of the immune system), and the second stage is a delayed inflammatory response mediated by TGF-β signaling.
- It is expected that the evaluation platform developed in this research will contribute to elucidating the mechanisms of drug-induced liver damage and developing new treatments and prevention methods that reduce side effects.
Outlines
A research group including Shuntaro Kawamura (doctoral student) of the Human Biology Research Unit, Institute of Integrated Research (IIR) of the Science Tokyo, Professor Takanori Takebe (Professor at the Graduate School of Medicine and Vice Director of the Premium Research Institute for Human Metaverse Medicine of the University of Osaka), and Assistant Professor Noriki Okada of the Department of Surgery at Jichi Medical University, has used human iPSC-derived vascularized liver organoids to elucidate in detail the mechanism of hepatic microvasculopathy caused by the immunosuppressive agent ATG.
ATG is widely used to suppress rejection after organ transplantation, including liver transplants but is known to sometimes cause serious side effects such as thrombus formation and liver dysfunction. However, the detailed mechanism of its onset has not been elucidated until now due to the lack of an appropriate model that can reproduce the blood flow in the human liver.
In this research, the research group transplanted human iPSC-derived vascularized liver organoids onto the surface of the brain of a mouse, and by utilizing AI, established a intravital imaging system that enables long-term observation of the dynamics of human blood vessels at high resolution while the animal model is alive. When ATG was administered using this system, it was discovered that the complement system was excessively activated on endothelial cells in human blood vessels, causing rapid thrombus formation (first stage). Furthermore, approximately 24 hours later, the TGF-β pathway was activated, and a delayed inflammatory response accompanied by the accumulation of neutrophils (a type of white blood cell) further exacerbated the vascular impairment (second stage).
Additionally, they analyzed liver tissues taken from actual patients who developed liver injuries due to ATG and confirmed pathological findings consistent with the phenomena observed in this model, demonstrating the clinical validity of this study.
These findings shed light for the first time on the mechanism of ATG-induced vascular impairment, which has been considered a black box until now. The model established in this research is expected to become a new platform to contribute to the development of safer immunosuppressive therapies and to elucidating the pathogenesis of various drug-induced liver injuries.
Fig. 1 Overview of the research results
By administering the immunosuppressive agent ATG to human liver organoids with sinusoidal after organ transplantation, the research group elucidated the mechanism by which thrombus formation progresses in stages
Credit: Takanori Takebe
Research Background
Antithymocyte globulin (ATG) is a potent immunosuppressive agent used to suppress rejection in organ transplants and to treat graft-versus-host disease (GVHD).
Although ATG is highly effective, it has been reported to cause thrombocytopenia, thrombosis, and even a severe liver injury called sinusoidal obstruction syndrome (SOS), which has been a clinical issue. It had been suggested that ATG acts on vascular endothelial cells, however, there was no way to directly observe what was happening in the tiny sinusoids in the human liver, and the detailed pathological mechanism remained unknown.
Research Contents
The research group transplanted human liver bud organoids (HLBOs), which were created by self-organizing hepatic parenchymal cells, mesenchymal stem cells (MSC), and endothelial progenitors from human iPSCs, onto the brain surface of immunodeficient mice. The endothelial progenitors contained in HLBO differentiated into liver sinusoidal endothelial cells (iLSECs), which are liver-specific vascular endothelial cells on the brain surface, and were able to reproduce the pathological condition of thrombosis that occurs in the liver sinusoids.
Because the brain surface is stable and moves little, confocal laser scanning microscopy makes it possible to observe the inner parts of the human-derived vascular network constructed within the organoids at high resolution and over time while the organoids are alive.
This model mouse was intravenously injected with clinically used concentrations of ATG, and changes within the blood vessels were observed. The result indicated the following two-stage pathology:
1. Stage 1: Complement-driven acute thrombus formation (up to 6 hours after administration)
Just 1 hour after ATG administration, the drug selectively bound to human iLSEC blood vessels, and 3 hours later, complement C3, which plays a key role in immune defense, was deposited on the surface of endothelial cells within the blood vessel.
This caused platelets to aggregate one after another, forming tiny clots that blocked blood flow.
This reaction was specific to human blood vessels and was not observed in the mouse's blood vessels.
Furthermore, when inhibitors are administered beforehand to inhibit complement function, ATG-induced thrombus formation was significantly suppressed, proving that complement activation is the main cause in the early stages.
Fig. 2 ATG-mediated complement-dependent thrombosis recreated in vascularized human liver organoids
A. Vascularization of iLSECs in liver organoids (HLBOs) in the mouse cranial window
B. Fluorescent images of iLSEC blood vessels (human CD31, blue), platelets (CD41, green), and ATG (red) stained in HLBO 1 hour after ATG administration.
C. Live imaging images taken 24 hours after ATG administration comparing control group and complement inhibitor treatment, visualizing blood (Dextran, blue), platelets (CD41, green), complement factor C3 (C3, red), and iLSECs (white).
Credit: Takanori Takebe
2. Stage 2: Delayed inflammation and vascular destruction via the TGF-β pathway (24 hours after administration)
Comprehensive analysis of gene expression in iLSECs 24 hours after ATG administration clarified that several genes (e.g., CCL2, SERPINE1) that promote inflammation and thrombosis were activated.
These genes are found to be controlled by TGF-β signaling pathway, which is involved in cell proliferation and fibrosis.
Actual bioimaging confirmed that at this point, neutrophils accumulate in human blood vessels, causing the blood vessel walls to break down and leak their contents, as vascular disruption.
Furthermore, administration of a drug (SB431542) that inhibits the motion of TGF-β improved thrombus formation and blood flow disorders, indicating that the TGF-β pathway contributes to the worsening of the pathological condition.
Fig. 3 Amplification of ATG-induced thrombosis through activation of the TGF-β pathway in iLSECs.
A. Live imaging showing the release of neutrophil elastase (NE, red) from neutrophils (Ly6G, green) bounding to iLSEC blood vessels (blue) 6 hours after ATG administration.
B. Live imaging taken 24 hours after ATG administration, comparing vehicle and TGF-β inhibitor treatments. Visualization of blood (Dextran, blue), neutrophils (Ly6G, green), platelets (CD41, red), and iLSECs (white).
C. Quantification of the area of thrombus in iLSEC blood vessels 24 hours after ATG administration (mean ± SD) comparing the area treated with vehicle and TGF-β inhibitor.
Credit: Takanori Takebe
To verify the findings obtained from these models, liver biopsies from patients who had developed SOS after ATG treatment were analyzed and confirmed that thrombus formation in the hepatic sinusoids, complement (C4d) deposition, and neutrophil accumulation were confirmed same as the model.
This demonstrates the clinical significance of the research results.
Social Impact of the Research
By combining human iPSC-derived organoids with bioimaging technology, this research succeeded in visualizing the dynamic process of drug side effects in human organs, something that was previously impossible.
The result of this study, which clarified that ATG-induced liver damage progresses in two stages: acute thrombosis caused by complement and delayed inflammation caused by TGF-β, is an important finding that will lead to the establishment of safer administration methods and the development of therapeutic drugs that target side effects.
Future Developments
The evaluation platform established in this study is not only effective for predicting the risk of liver damage caused by ATG, but is also expected to be a potent tool that can be applied to elucidating the pathology of various vascular-related diseases, such as liver injury caused by other drugs and thrombotic microangiopathy, as well as in drug discovery research.
Notes
The article, “Modeling antithymocyte globulin-induced microvasculopathy using human iPSC-derived vascularized liver organoids,” was published in Cell Reports Medicine at DOI: 10.1016/j.xcrm.2025.102433
