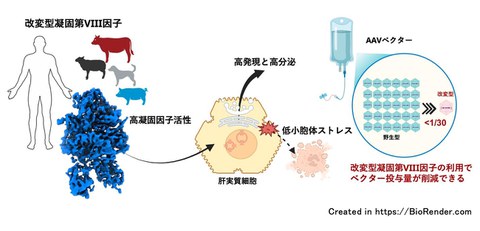
Advancing domestic hemophilia A gene therapy
Developed engineered factor VIII (FVIII) with enhanced functions
- Engineered coagulation factor VIII (FVIII) was developed by referring to the amino acid sequences of blood coagulation FVIII from various animal species.
- The engineered coagulation FVIII has enhanced various functions, including high activity, secretion ability, and significantly reduced endoplasmic reticulum stress response.
- In a test using cynomolgus monkeys, an increase in FVIII activity exceeding the therapeutic range (reference value) was achieved with one-thirtieth of the dose used for Roctavian®, a gene therapy drug approved and sold overseas.
Effective hemophilia A gene therapy using engineered FVIII
Credit: Susumu Uchiyama
Outlines
A research group including Associate Professor Yuji Kashiwakura and Professor Tsukasa Ohmori of the Center For Gene Therapy Research at School of Medicine of Jichi Medical University, Professor Keiji Nogami of the Department of Pediatrics, Nara Medical University, Professor Osamu Nureki of the Graduate School of Science, The University of Tokyo, Dr. Tiago Lopes of Nezu Life Sciences (now Nezu Biotech GmbH), Professor Susumu Uchiyama of the Graduate School of Engineering, the University of Osaka, and the Corporation for Production and Research of Laboratory Primates, has succeeded in developing a highly functional engineerd blood coagulation factor VIII (FVIII) that dramatically increases activity of coagulation factor and secretion capacity and reduces endoplasmic reticulum stress, by comparing the amino acid sequence of blood coagulation FVIII across animal species. These results suggest that treatment may be possible with a significantly lower vector dose than the existing hemophilia A gene therapy drug (Roctavian®) that uses adeno-associated virus (AAV) vectors. If this high-function FVIII can reduce the dosage of drugs for hemophilia A gene therapy, it could reduce both treatment costs and risk of side effects, potentially increasing the practicality and safety of the drug.
Research Background
Hemophilia A is a bleeding disorder caused by a deficiency or functional decline of the FVIII gene in the blood. Treatments include replenishing the coagulation factor proteins that are lacking in the blood and antibody drugs that replace the function of FVIII. However, patients in critical condition must continue medication for life. Recently, gene therapy drugs using AAV vectors have been approved in Europe and the United States, and it is expected that a single administration will provide long-term therapeutic effects.
Meanwhile, hemophilia A gene therapy requires a large dose of vector, and the therapeutic effect peaks one year after administration before gradually weakening, suggesting that the long-term therapeutic effect is limited. Considering the need to ensure safety and the cost of AAV vector production, it is important to find ways to reduce the dosage required for treatment. Furthermore, to sustain the effectiveness of hemophilia A gene therapy, it is also important to reduce the induction of endoplasmic reticulum stress caused by protein expression, which is thought to be one of the major factors.
Hemophilia B gene therapy uses a protein that enhances the function of the deficient blood coagulation factor IX (Padua mutant) which is a highly active blood coagulation factor IX found in patients with thrombosis and is known to have approximately eight times higher coagulation factor function than normal. By using this mutant, it became possible to reduce the amount of AAV vector administered in hemophilia B gene therapy. Therefore, in this study, the researchers attempted to develop an engineered coagulation factor VIII with enhanced coagulation factor activity and secretion capacity, as well as a low endoplasmic reticulum stress response, in order to enable gene therapy for hemophilia A through stable gene expression with low vector dosage.
Research Contents
To date, it has been known that non-human mammals have higher FVIII activity than humans. In this study, the research group focused on amino acid sequences in various non-human mammals and identified an engineered FVIII with 36 amino acid substitution sites. The identified engineered FVIII showed eight times the coagulation factor activity and four times the secretion capacity compared to the wild-type FVIII. Furthermore, in a study using a liver cell line, intracellular FVIII accumulation was significantly reduced, and the endoplasmic reticulum stress response was significantly attenuated.
Coagulation factor expression increased in hemophilia A mice induced by AAV vector carrying engineered FVIII
Credit: Susumu Uchiyama
Biochemical analysis of the engineered FVIII protein clarified the fact that its activity was enhanced by improving its affinity for activated coagulation factor IX, and that the dissociation of the A2 domain indicating inactivation was promoted. Furthermore, analysis conducted after translational modifications indicated the possibility that new glycosylation sites may improve intracellular transport efficiency.
Structural analysis using cryogenic electron microscopy revealed that although the overall structure of FVIII did not show significant change, it was possible to explain the enhanced binding with activated factor IX due to increased non-covalent bonds and the separation of the A2 domain due to instability between the domains. With engineered proteins, there is concern about antigenicity due to amino acid substitutions. In silico binding analysis with major human HLA class II did not confirm any new high-affinity epitopes due to the modification, and BCR Repertoire Analysis in mice that developed inhibitors (neutralizing antibodies) to FVIII also showed that the immunogenicity risk of modified FVIII was similar to that of wild-type FVIII. Furthermore, when the genetically optimized AAV vector was administered to cynomolgus monkeys, an increase in FVIII activity exceeding the reference range was confirmed even at a dose of 2 x 10¹² vg/kg—one-thirtieth of the dose used for Roctavian®, a gene therapy drug marketed in Europe and the United States.
Biochemical characteristics of engineered FVIII
Credit: Susumu Uchiyama
The Importance of This Study
This study demonstrated that the high activity and high secretion of engineered FVIII enable hemophilia A gene therapy using low-dose vectors, significantly reducing the risk of side effects. Furthermore, reduced endoplasmic reticulum stress induction may enable long-term sustained expression of FVIII. This achievement represents a technological foundation that could dramatically improve the safety and persistence of AAV vector for hemophilia A gene therapy, and marks a significant step forward in the practical application of gene therapy drugs for hemophilia A.
Notes
The article, “Engineered coagulation factor VIII with enhanced secretion and coagulation potential for hemophilia A gene,” was published in Blood at DOI: https://doi.org/10.1182/blood.2025028481.
