\Confirmed to suppress relapses!/ Rituximab brings new hope for adult patients with frequently relapsing nephrotic syndrome
- It has been demonstrated that the antibody preparation rituximab is an effective and safe treatment for preventing relapses and maintaining remission in adult-onset frequently relapsing/steroid-dependent nephrosis by conducting a double-blind, randomized trial.
- In frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome, long-term use of steroids to treat relapses can lead to complications such as osteoporosis and infections. Until now, rituximab has been covered by insurance as a treatment for patients with childhood onset, but no evidence has been obtained to support its coverage for adult onset.
- The results of the trial indicated that the 49-week relapse-free rate, the primary endpoint, was 87.4% in the rituximab group and 38.0% in the placebo group, demonstrating that rituximab suppresses relapses.
- It is expected that rituximab will be covered by insurance as an effective and safe treatment for preventing relapses and maintaining remission also in adult-onset frequently relapsing/steroid-dependent nephrosis.
Outlines
A research group including Professor Yoshitaka Isaka and Assistant Professor Yusuke Sakaguchi of the Department of Nephrology, Graduate School of Medicine of the University of Osaka, and Lecturer Maki Shinzawa (at the time of the research: Specially Appointed Assistant Professor) of the University's Health and Counseling Center, demonstrated that the antibody preparation rituximab is an effective and safe treatment for preventing relapses and maintaining remission in adult-onset frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome by conducting a double-blind, randomized trial. This study was an investigator-initiated clinical trial conducted at 13 centers in Japan (University of Osaka, Nagoya University, Kindai University, Fujita Health University, Kurume University, Niigata University, Kitano Hospital, Saitama Medical University, University of Tokyo, Kanazawa University, Toranomon Hospital, Kanazawa Medical University, and University of Tsukuba).
Rituximab, a type of lymphocyte, is an antibody preparation that suppresses B-cells. Until now, it has mainly been used for childhood-onset frequently relapsing/steroid-dependent nephrotic syndrome, but its efficacy and safety in adult-onset patients have also been confirmed by conducting a double-blind, randomized trial.
The trial involved adult-onset, frequently relapsing/steroid-dependent nephrosis patients, divided into a rituximab group (32 patients) and a placebo group (34 patients), and compared the results of 49 weeks of treatment. The results of the trial indicated that the 49-week relapse-free rate, the primary endpoint, was 87.4% in the rituximab group, and 38.0% in the placebo group, demonstrating that rituximab suppresses relapses. In addition, the proportion of patients who were able to discontinue steroids at 49 weeks was significantly higher in the rituximab group, and the incidence of serious adverse events occurred in 3.1% and 2.9% of patients in the rituximab and placebo groups, respectively.
In frequently relapsing/steroid-dependent nephrotic syndrome, long-term use of steroids can lead to complications such as osteoporosis and infections, which can have a negative impact on patients' quality of life, survival rates, and health. The results of this study show that rituximab may be an effective and safe treatment for preventing relapses and maintaining remission in such patients.
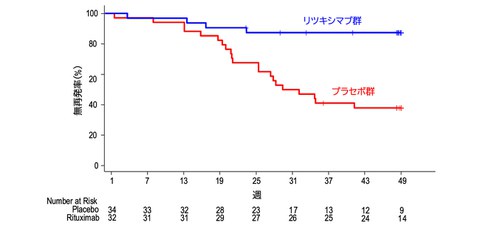
Fig. 1 Relapse-free rate during the double-blind period (Kaplan-Meier method)
Credit: Yoshitaka Isaka
Research Background
Nephrotic syndrome is a disease characterized by severe proteinuria and hypoalbuminemia. Although minimal-change nephrotic syndrome that develops in adults can be remitted in approximately 90% of cases with steroid treatment, approximately half of these patients experience relapses during steroid tapering.
Many of them experience repeated relapses (frequent relapses) and are unable to reduce the steroid dose because the disease relapses when the steroid is tapered (steroid dependence). Also, they develop complications such as osteoporosis and infections due to long-term steroid use, which negatively impact their quality of life and survival rate.
Rituximab has indicated its effectiveness in preventing relapses in patients with childhood-onset frequently relapsing/steroid-dependent nephrosis and has been covered by insurance, but it has not been approved for use in adult-onset nephrosis patients, and evidence to obtain insurance coverage has been eagerly awaited.
Research Contents
Between September 1, 2020, and June 28, 2022, a double-blind, randomized, placebo-controlled trial was conducted in 72 patients with adult-onset frequently relapsing/steroid-dependent nephrosis. They were randomized to receive rituximab (36 patients) or placebo (36 patients). Of those, six patients withdrew before receiving the investigational drugs, leaving 66 (rituximab group: 32; placebo group: 34) who received the investigational drugs during the double-blind period.
The 49-week relapse-free rate during the double-blind period was 87.4% (95% CI, 69.8%-95.1%) in the rituximab group and 38.0% (22.1%-53.8%) in the placebo group. Median times to relapse were 49.0 weeks in the rituximab group and 30.8 weeks in the placebo group and the hazard ratio (HR) for recurrence in the rituximab group was 0.16 (0.05–0.46) (Fig. 1). The proportions of patients who discontinued corticosteroids at 49 weeks were 71.9% and 36.4% in the rituximab and placebo groups, respectively (P = 0.0061). These findings demonstrate that rituximab is effective in discontinuing steroids, preventing relapses, and maintaining remission in patients with adult-onset frequently relapsing/steroid-dependent nephrosis.
In the rituximab group, 4 of 32 patients relapsed during the double-blind period, of which 3 patients entered the post-relapse treatment phase. In the placebo group, 22 of 34 patients relapsed during the double-blind period, of which 20 patients entered the post-relapse treatment phase.
The overall relapse-free rates at week 49 were 95.7% (CI, 72.9%-99.4%) in the rituximab relapse group and 100% (CI, 100%-100%) in the placebo relapse group. (Fig. 2)
Although rituximab is a drug that suppresses B-cells, which are lymphocytes, drug-related serious adverse events occurred in 3.1% and 2.9% of patients in the rituximab and placebo groups, respectively. Furthermore, this study was conducted during a time when COVID-19 was raging, and 15.6% of patients in the rituximab group and 5.9% of patients in the placebo group were infected with it, but no patients developed serious symptoms.
Fig. 2 Number of patients with remission maintenance and relapse during the blinded and treatment periods
Credit: Yoshitaka Isaka
Social Impact of the Research
Patients with adult-onset frequently relapsing/steroid-dependent nephrotic syndrome have been facing problems with side effects caused from long-term steroid administration. Rituximab has been shown to be effective in preventing relapses in patients with childhood-onset frequently relapsing/steroid-dependent nephrosis and is covered by insurance, but it has not been approved for use in adult-onset, and evidence to obtain insurance coverage has been eagerly awaited.
The research group has demonstrated the efficacy and safety of rituximab and is now in discussion with the PMDA regarding its insurance coverage.
Notes
The article, “Rituximab for relapsing nephrotic syndrome in adults; A Randomized Clinical Trial,” was published in American scientific journal of JAMA at DOI: https://doi.org/10.1001/jama.2025.19316 .
